内藤 幹彦
Teamケミカルプロテインノックダウン技術の開発と細胞制御
研究代表者 内藤 幹彦東京大学大学院薬学系研究科 特任教授
|
研究概要
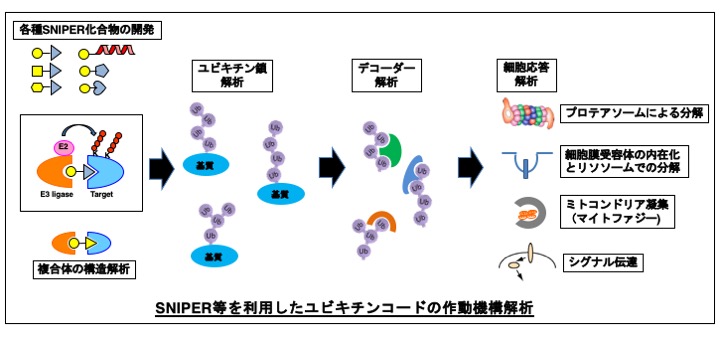
ユビキチン・プロテアソーム系は選択的タンパク質分解経路の一つで、細胞内で不要になったタンパク質等はユビキチンリガーゼ(E3)によって認識され、ユビキチン修飾を受けた後プロテアソームで分解されます。またユビキチン修飾はタンパク質分解以外にも様々な細胞機能制御に関与し、これにはユビキチン鎖の連結様式、鎖長、分岐などの違いによる多様性(ユビキチンコード)が重要であると考えられていますが、その詳細はまだよくわかっていません。
私たちは、SNIPER(Specific and Nongenetic IAP-dependent Protein Eraser)やセレブロン(CRBN)モジュレーター等の化合物を利用して細胞内の標的タンパク質をユビキチン化し、プロテアソームで分解するケミカルプロテインノックダウン技術を開発してきました。SNIPERはIAPに結合するリガンド(IAPアンタゴニスト)と標的タンパク質に結合するリガンド(分子弾頭)を繋いだキメラ化合物で、標的タンパク質にE3活性を持つIAPをリクルートする事によって標的タンパク質ユビキチン化してプロテアソームで分解します。分子弾頭を置換することによって任意の標的タンパク質を狙って分解する事ができる汎用性の高い技術です。一方CRBNモジュレーターは複合体型E3の基質認識分子であるCRBNに結合して基質特異性を変化させる事ができます。最近これらの化合物が、細胞膜受容体の内在化を誘導するなど、ユビキチンが関与するタンパク質分解以外の様々な細胞応答を引き起こすことがわかってきました。
本研究では、これらの化合物を利用して様々な標的タンパク質をユビキチン化し、そのユビキチン鎖と細胞応答等を解析することによって、ユビキチンコードの作動機構の一端を明らかにすることを目的としています。また本研究で新たに開発する化合物は新しい分子標的薬のリードとなる可能性があり、将来的には創薬にも貢献する有意義な研究になると考えています。
研究概要を示す模式図
本領域での研究成果
- Shibata N, Shimokawa K, Nagai K, Ohoka N, Hattori T, Miyamoto N, Ujikawa O, Sameshima T, Nara H, Cho N, Naito M.
Pharmacological difference between degrader and inhibitor against oncogenic BCR-ABL kinase.
Sci. Rep. 8, 13549 (2018)
PMID: 30202081 - Misawa T, Goto C, Shibata N, Hirano M, Kikuchi Y, Naito M, Demizu Y.
Rational design of novel amphipathic antimicrobial peptides focused on distribution of cationic amino acid residues.
MedChemComm 10,896-900 (2019)
PMID: 31303986 - Ohoka N, Ujikawa O, Shimokawa K, Sameshima T, Shibata N, Hattori T, Nara H, Cho N, Naito M.
Different Degradation Mechanisms of Inhibitor of Apoptosis Proteins (IAPs) by the Specific and Nongenetic IAP-Dependent Protein Eraser (SNIPER).
Chem. Pharm. Bull. 67, 203-209 (2019)
PMID: 30369550 - Misawa T, Ohoka N, Oba M, Yamashita H, Tanaka M, Naito M, Demizu Y.
Development of 2-aminoisobutyric acid (Aib)-rich cell-penetrating foldamers for efficient siRNA delivery.
Chem. Commun. 55,7792-7795 (2019)
PMID: 31210205 - Ohoka N, Tsuji G, Shoda T, Fujisato T, Kurihara M, Demizu Y, Naito M.
Development of Small Molecule Chimeras That Recruit AhR E3 Ligase to Target Proteins.
ACS Chem. Biol. 14, 2822-2832 (2019)
PMID: 31580635 - *Shoda T, Ohoka N, Tsuji G, Fujisato T, Inoue H, Demizu Y, Naito M, Kurihara M.
Targeted protein degradation by chimeric compounds using hydrophobic E3 ligands and adamantane moiety.
Pharmaceuticals 13, 34 (2020)
PMID: 32106507 - Shibata N, Ohoka N, Tsuji G, Demizu Y, Akiyama T, *Naito M.
Deubiquitylase USP25 prevents degradation of BCR-ABL protein and ensures proliferation of Ph-positive leukemia cells.
Oncogene 39, 3867-3878 (2020)
PMID: 32203161 - Yokoo H, Ohoka N, Naito M, *Demizu Y.
Design and synthesis of peptide-based chimeric molecules to induce degradation of the estrogen and androgen receptors.
Bioorg. Med. Chem. 28, 115595 (2020)
PMID: 32631565 - *Yamano K, Kikuchi R, Kojima W, Hayashida R, Koyano F, Kawakami J, Shoda T, Demizu Y, Naito M, Tanaka K, Matsuda N.
Critical role of mitochondrial ubiquitination and the OPTN-ATG9A axis in mitophagy.
J. Cell Biol. 219, e201912144 (2020)
PMID: 32556086 - Yokoo H,# Shibata N,# Naganuma M, Murakami Y, Ito T, *Aritake K, *Naito M, *Demizu Y.
Development of hematopoietic prostaglandin D synthase-degradation inducer.
ACS Med. Chem. Lett. 12,236-241 (2021)
PMID: 33603969
#These authors equally contributed to this work.
<Selected as Supplementary Cover> - Soma-Kaiho A, Akizuki Y, Igarashi K, Endo A, Kawase Y, Shoda T, Demizu Y, Naito M, Saeki Y, Tanaka K, *Ohtake F.
TRIP12 enhances small molecule-induced degradation of BRD4 through K29/K48 branched ubiquitin chains.
Mol. Cell. 81, 1411-1424.e7 (2021)
PMID: 33567268
- Tsukumo Y,# Tsuji G,# Yokoo H, Shibata N, Ohoka N, Demizu Y, *Naito M.
Protocols for synthesis of SNIPERs and the methods to evaluate the anticancer effects.
Methods Mol. Biol. 2365, 331-347 (2021)
PMID: 34432253
#These authors equally contributed to this work. - Xu H,# Ohoka N,# Yokoo H, Nemoto K, Ohtsuki T, Matsufuji H, Naito M, Inoue T, *Tsuji G, *Demizu Y.
Development of agonist-based PROTACs targeting liver X receptor.
Front. Chem. 9, 674967 (2021)
PMID: 34124002
#These authors equally contributed to this work. - Yokoo H,# Ohoka N,# Takyo M, Ito T, Tsuchiya K, Kurohara T, Fukuhara K, Inoue T, Naito M, *Demizu Y.
Peptide stapling improves the sustainability of a peptide-based chimeric molecule that induces targeted protein degradation.
Int. J. Mol. Sci.22, 8772 (2021)
PMID: 34445478 - Yokoo H, *Shibata N, Endo A, Ito T, Yanase Y, Murakami Y, Fujii K, Hamamura K, Saeki Y, *Naito M, *Aritake K, *Demizu Y.
Discovery of a highly potent and selective PROTAC targeting hematopoietic prostaglandin D synthase via in silico design.
J. Med. Chem. 64, 15868-15882 (2021)
<Selected as Supplementary Cover> - Naganuma M, *Ohoka N, Tsuji G, Tsujimura H, Matsuno K, Inoue T, Naito M, *Demizu Y.
Development of chimeric molecules that degrade the estrogen receptor using decoy oligonucleotide ligands.
ACS Med. Chem. Lett. 13, 134-139 (2022)
PMID: 35059133
<Selected as Supplementary Cover> - #Ohoka N,#Yokoo H, Okuhira K,Demizu Y, *Naito M.
Molecular design, synthesis and evaluation of SNIPER(ER) that induces targeted protein degradation of ERα.
Methods Mol. Biol. 2418, 363-382 (2022)
PMID: 35119675
#These authors equally contributed to this work. - Shibata N, Cho N, Koyama H, Naito M.
Development of a degrader against oncogenic fusion protein FGFR3-TACC3.
Bioorg Med Chem Lett 60, 128584 (2022)
PMID: 35085722 - Yu S, Wang L, Che D, Zhang M, Li M, Naito M, Xin W, Zhou L.
Targeting CRABP-II overcomes pancreatic cancer drug resistance by reversing lipid raft cholesterol accumulation and AKT survival signaling.
J Exp Clin Cancer Res 41, 88 (2022)
PMID: 35260193 - Nakano N, Fukuda K, Tashiro E, Ishikawa H, Nagano W, Kawamoto R, Mori A, Watanabe M, Yamazaki R, Nakane T, Naito M, Okamoto I, *Itoh, S.
Hybrid molecule between platanic acid and LCL-161 as a yes-associated protein degrader.
J Biochem. 171, 631-640 (2022)
PMID: 35211741 - #Xu H, #Kurohara T, Takano R, Yokoo H, Shibata N, Ohoka N, Inoue T, Naito M, *Demizu Y.
Development of rapid and facile solid-phase synthesis of PROTACs via a variety of binding styles.
ChemistryOpen. 11, e202200131 (2022)
PMID: 35822913
#These authors equally contributed to this work. - Ohoka N, Suzuki M, Uchida T, Tsukumo Y, Yoshida M, Inoue T, Ohki H, *Naito M.
Development of a potent small-molecule degrader against oncogenic BRAF(V600E) protein that evades paradoxical MAPK activation.
Cancer Sci. 113, 2828-2838 (2022)
PMID: 35579105 - #Murakami Y, #Osawa H, #Kurohara T, Yanase Y, Ito T, Yokoo H, Shibata N, Naito M, Aritake K, *Demizu Y.
Structure-activity relationship study of PROTACs against hematopoietic prostaglandin D2 synthase.
RSC Med. Chem. 13, 1495-1503 (2022)
PMID: 36561070
#These authors equally contributed to this work. <Selected as Front Cover> - Akizuki Y, Morita M, Mori Y, Kaiho-Soma A, Dixit S, Endo A, Shimogawa M, Hayashi G, Naito M, Okamoto A, Tanaka K, Saeki Y, *Ohtake F.
cIAP1-based degraders induce degradation via branched ubiquitin architectures.
Nat Chem Biol. 19, 311-322 (2022)
PMID: 36316570 - Ohoka N, Suzuki M, Uchida T, Tsuji G, Tsukumo Y, Yoshida M, Inoue T, Demizu Y, Ohki H, *Naito M.
Development of Gilteritinib-Based Chimeric Small Molecules that Potently Induce Degradation of FLT3-ITD Protein.
ACS Med. Chem. Lett. 13, 1885-1891 (2022)
PMID: 36518702
代表的な論文
- Itoh Y, Ishikawa M, *Naito M, *Hashimoto Y.
Protein knockdown using methyl bestatin-ligand hybrid molecules: design and synthesis of inducers of ubiquitination-mediated degradation of cellular retinoic acid-binding proteins.
J. Am. Chem. Soc. 132, 5820-5826 (2010)
PMID: 20369832 - Ohoka N, Okuhira K, Ito M, Nagai K, Shibata N, Hattori T, Ujikawa O, Shimokawa K, Sano O, Koyama R, Fujita H, Teratani M, Matsumoto H, Imaeda Y, Nara H, Cho N, *Naito M.
In vivo knockdown of pathogenic proteins via specific and nongenetic inhibitor of apoptosis protein (IAP)-dependent protein erasers (SNIPERs).
J. Biol. Chem. 292, 4556-4570 (2017)
PMID: 28154167 - Shibata N, Nagai K, Morita Y, Ujikawa O, Ohoka N, Hattori T, Koyama R, Sano O, Imaeda Y, Nara H, Cho N, *Naito M.
Development of protein degradation inducers of androgen receptor by conjugation of androgen receptor ligands and inhibitor of apoptosis protein ligands.
J. Med. Chem. 61, 543-575 (2018)
PMID: 28594553 - Ohoka N, Morita Y, Nagai K, Shimokawa K, Ujikawa O, Fujimori I, Ito M, Hayase Y, Okuhira K, Shibata N, Hattori T, Sameshima T, Sano O, Koyama R, Imaeda Y, Nara H, Cho N, *Naito M.
Derivatization of inhibitor of apoptosis protein (IAP) ligands yields improved inducers of estrogen receptor α degradation.
J. Biol. Chem. 293, 6776-6790 (2018)
PMID: 29545311 - *Naito M, Ohoka N, Shibata N.
SNIPERs-Hijacking IAP activity to induce protein degradation.
Drug Discov. Today: Technol. 31, 35-42 (2019)
PMID: 31200857





