出水 庸介
Team中分子化合物による細胞機能制御
研究分担者 出水 庸介国立医薬品食品衛生研究所 有機化学部 部長 |
研究概要
タンパク質は精密な立体構造を形成することによって多種多様な機能を発現していますが、その立体構造の中でヘリカル構造はDNAや他のタンパク質を認識する重要な二次構造です。よってタンパク質の機能をペプチド単位で発現させるためには、タンパク質中と同じ安定なヘリカル構造を形成できるペプチド分子を設計することが重要です。私たちは、さまざまな非天然型アミノ酸や共有結合型のアミノ酸側鎖架橋(ステープル)を利用することで安定なヘリカル構造を形成できるペプチドの開発を行ってきました。
本研究では、これまでに開発してきたペプチド二次構造制御技術をケミカルプロテインノックダウンに応用することで中分子ペプチドによる細胞内タンパク質の精密制御を目指します。また、領域内のユビキチン研究を支援するために中分子ペプチド(ステープルペプチド)の供給を行います。さらに、中分子ペプチドと並び創薬モダリティのひとつであるオリゴ核酸を標的タンパク質のリガンドとして利用したケミカルプロテインノックダウン技術を開発します。
1. 二次構造制御ペプチドによる細胞制御
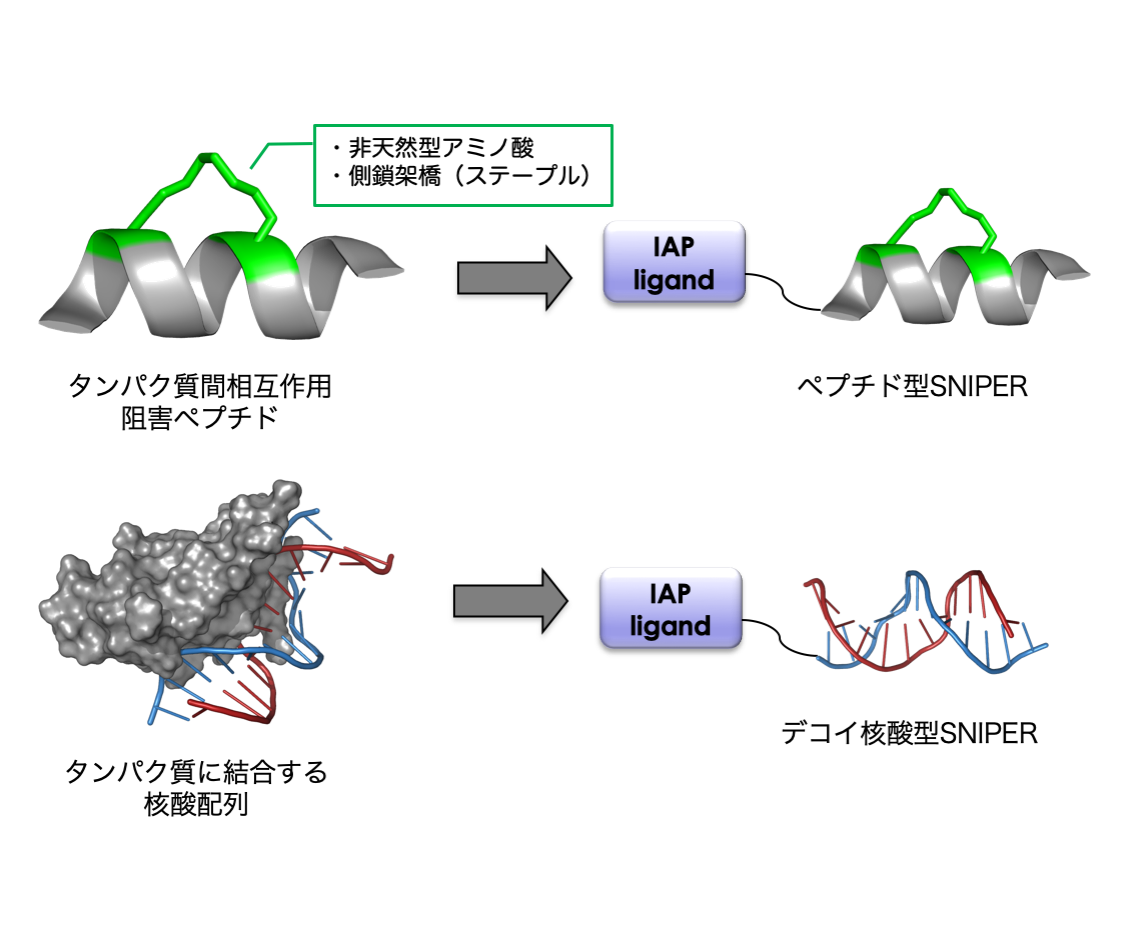
タンパク質間相互作用に基づいて安定な二次構造を形成できる高親和性ペプチド(ステープルペプチド等)を設計して、ペプチド型SNIPERの開発を行います。ペプチド型SNIPERは、低分子リガンドでは標的にすることが難しいタンパク質やリガンドが存在しないタンパク質をターゲットにすることができます。
2. オリゴ核酸による細胞制御
c-Mycや核内受容体等の転写因子を標的とするために、それらに結合する核酸配列をリガンドとして利用したデコイ核酸型SNIPERを開発します。本手法により、低分子化合物やペプチドでは標的にすることが困難なタンパク質をノックダウンできます。
研究概要を示す模式図
本領域での研究成果
- Misawa T, Goto C, Shibata N, Hirano M, Kikuchi Y, Naito M, Demizu Y.
Rational design of novel amphipathic antimicrobial peptides focused on distribution of cationic amino acid residues.
MedChemComm 10, 896-900 (2019)
PMID: 31303986 - Misawa T, Ohoka N, Oba M, Yamashita H, Tanaka M, Naito M, Demizu Y.
Development of 2-aminoisobutyric acid (Aib)-rich cell-penetrating foldamers for efficient siRNA delivery.
Chem. Commun. 55, 7792-7795 (2019)
PMID: 31210205 - Ohoka N, Tsuji G, Shoda T, Fujisato T, Kurihara M, Demizu Y, Naito M.
Development of Small Molecule Chimeras That Recruit AhR E3 Ligase to Target Proteins.
ACS Chem. Biol. 14, 2822-2832 (2019)
PMID: 31580635 - Goto C, Hirano M, Hayashi K, Kikuchi Y, Hara-Kudo Y, Misawa T, Demizu Y.
Development of amphipathic antimicrobial peptide foldamers based on Magainin 2 sequence.
ChemMedChem 14, 1911-1916 (2019)
PMID: 31667994 - *Shoda T, Ohoka N, Tsuji G, Fujisato T, Inoue H, Demizu Y, Naito M, Kurihara M.
Targeted protein degradation by chimeric compounds using hydrophobic E3 ligands and adamantane moiety.
Pharmaceuticals 13, 34 (2020)
PMID: 32106507 - Shibata N, Ohoka N, Tsuji G, Demizu Y, Akiyama T, *Naito M.
Deubiquitylase USP25 prevents degradation of BCR-ABL protein and ensures proliferation of Ph-positive leukemia cells.
Oncogene 39, 3867-3878 (2020)
PMID: 32203161 - Yokoo H, Ohoka N, Naito M, *Demizu Y.
Design and synthesis of peptide-based chimeric molecules to induce degradation of the estrogen and androgen receptors.
Bioorg. Med. Chem. 28, 115595 (2020)
PMID: 32631565 - *Yamano K, Kikuchi R, Kojima W, Hayashida R, Koyano F, Kawakami J, Shoda T, Demizu Y, Naito M, Tanaka K, Matsuda N.
Critical role of mitochondrial ubiquitination and the OPTN-ATG9A axis in mitophagy.
J. Cell Biol. 219, e201912144 (2020)
PMID: 32556086 - Hirano M, Saito C, Goto C, Yokoo H, Kawano R, *Misawa T, *Demizu Y.
Rational design of helix-stabilized antimicrobial peptide foldamers containing a,a-disubstituted amino acids or side-chain stapling.
ChemPlusChem 85,2731-2736 (2020)
PMID: 33369262 - Yokoo H,# Shibata N,# Naganuma M, Murakami Y, Ito T, *Aritake K, *Naito M, *Demizu Y.
Development of hematopoietic prostaglandin D synthase-degradation inducer.
ACS Med. Chem. Lett. 12,236-241 (2021)
PMID: 33603969
#These authors equally contributed to this work.
<Selected as Supplementary Cover> - Hirano M, Saito C, Yokoo H, Goto C, Kawano R, *Misawa T, *Demizu Y.
Structure-activity relationship analysis of Mag2-based peptide foldamers.
Molecules 26,444 (2021)
PMID: 33466998 - Soma-Kaiho A, Akizuki Y, Igarashi K, Endo A, Kawase Y, Shoda T, Demizu Y, Naito M, Saeki Y, Tanaka K, *Ohtake F.
TRIP12 enhances small molecule-induced degradation of BRD4 through K29/K48 branched ubiquitin chains.
Mol. Cell. 81, 1411-1424.e7 (2021)
PMID: 33567268 - Tsukumo Y,# Tsuji G,# Yokoo H, Shibata N, Ohoka N, Demizu Y, *Naito M. Protocols for synthesis of SNIPERs and the methods to evaluate the anticancer effects.
Methods Mol. Biol. 2365, 331-347 (2021)
PMID: 34432253
#These authors equally contributed to this work. - Xu H,# Ohoka N,# Yokoo H, Nemoto K, Ohtsuki T, Matsufuji H, Naito M, Inoue T, *Tsuji G, *Demizu Y.
Development of agonist-based PROTACs targeting liver X receptor.
Front. Chem. 9, 674967 (2021)
PMID: 34124002
#These authors equally contributed to this work. - *Yokoo H, Hirano M, Ohoka N, Misawa T, *Demizu Y.
Structure-activity relationship study of amphipathic antimicrobial peptides using helix-destabilizing sarcosine.
J. Pept. Sci. e3360, 1-6 (2021)
PMID: 34164880 - Yokoo H,# Ohoka N,# Takyo M, Ito T, Tsuchiya K, Kurohara T, Fukuhara K, Inoue T, Naito M, *Demizu Y.
Peptide stapling improves the sustainability of a peptide-based chimeric molecule that induces targeted protein degradation.
Int. J. Mol. Sci.22, 8772 (2021)
PMID: 34445478 - Yokoo H, *Shibata N, Endo A, Ito T, Yanase Y, Murakami Y, Fujii K, Hamamura K, Saeki Y, *Naito M, *Aritake K,
*Demizu Y. Discovery of a highly potent and selective PROTAC targeting hematopoietic prostaglandin D synthase via in silico design.
J. Med. Chem. 64, 15868-15882 (2021)
<Selected as Supplementary Cover> - Naganuma M, *Ohoka N, Tsuji G, Tsujimura H, Matsuno K, Inoue T, Naito M, *Demizu Y.
Development of chimeric molecules that degrade the estrogen receptor using decoy oligonucleotide ligands.
ACS Med. Chem. Lett. 13, 134-139 (2022)
PMID: 35059133
<Selected as Supplementary Cover> - #Ohoka N, #Yokoo H, Okuhira K, Demizu Y, *Naito M.
Molecular design, synthesis and evaluation of SNIPER(ER) that induces targeted protein degradation of ERα.
Methods Mol. Biol. 2418, 363-382 (2022)
PMID: 35119675
#These authors equally contributed to this work. - #Xu H, #Kurohara T, Takano R, Yokoo H, Shibata N, Ohoka N, Inoue T, Naito M, *Demizu Y.
Development of rapid and facile solid-phase synthesis of PROTACs via a variety of binding styles.
ChemistryOpen. 11, e202200131 (2022)
PMID: 35822913
#These authors equally contributed to this work. - Takada H, Tsuchiya K, *Demizu Y.
Helix-stabilized cell-penetrating peptides for delivery of antisense morpholino oligomers: Relationships among helicity, cellular uptake, and antisense activity.
Bioconjug. Chem. 33, 1311-1318 (2022)
PMID: 35737901
<Selected as Front Cover> - #Murakami Y, #Osawa H, #Kurohara T, Yanase Y, Ito T, Yokoo H, Shibata N, Naito M, Aritake K, *Demizu Y.
Structure-activity relationship study of PROTACs against hematopoietic prostaglandin D2 synthase.
RSC Med. Chem. 13, 1495-1503 (2022)
PMID: 36561070
#These authors equally contributed to this work. <Selected as Front Cover> - #Yokoo H, #Misawa T, Kato T, Tanaka M, *Demizu Y, *Oba M.
Development of delivery carriers for plasmid DNA by conjugation of a helical template to oligoarginine.
Bioorg. Med. Chem. 72, 116997 (2022)
PMID: 36088811
#These authors equally contributed to this work. - Tsuchiya K, Kiyoshi M, Hashii N, Fujita M, Kurohara T, Ishii-Watabe A, Fukuhara K, *Misawa T, *Demizu Y.
Development of a penetratin-conjugated stapled peptide that inhibits Wnt/β-catenin signaling.
Bioorg. Med. Chem. 73, 117021 (2022)
PMID: 36198218 - Takyo M, Sato Y, Hirata N, Tsuchiya K, Ishida H, Kurohara T, Yanase Y, Ito T, Ito N, Kanda Y, Yamamoto K, *Misawa T, *Demizu Y.
Oligoarginine-conjugated peptide foldamers inhibiting vitamin D receptor-mediated transcription.
ACS Omega. 7, 46573-46582 (2022)
PMID: 36570290
代表的な論文
- *出水庸介,三澤隆史,*栗原正明:「短鎖ペプチドのヘリカル構造制御と機能化」有機合成化学協会誌,72巻12号1336-1347(2014)
- Yamashita H, Kato T, Oba M, Misawa T, Hattori T, Ohoka N, Tanaka M, *Naito M, *Kurihara M, *Demizu Y.
Development of a cell-penetrating peptide that exhibits responsive changes in its secondary structure in the cellular environment.
Sci. Rep. 6, 33003 (2016)
PMID: 27609319 - Kobayashi H, Misawa T, Matsuno K, *Demizu Y.
Preorganized cyclic α,α-disubstituted α-amino acids bearing functionalized side chains that act as peptide-helix inducers.
J. Org. Chem. 82, 10722-10726 (2017)
PMID: 28915041 - *Misawa T, Kanda Y, *Demizu Y.
Rational design and synthesis of post-functionalizable peptide foldamers as helical templates.
Bioconj.Chem. 28, 3029-3035 (2017)
PMID: 29136364 - Okitsu K, Hattori T, Misawa T, Shoda T, Kurihara M, *Naito M, *Demizu Y.
Development of a small hybrid molecule that mediates degradation of His-tag fused proteins.
J. Med. Chem. 61, 576-582 (2018)
PMID: 28460164





