Tsukasa Okiyoneda
Elucidation of the mechanism of plasma membrane protein quality control by RFFL and its application to CF drug discovery
 |
Tsukasa Okiyoneda, PhDDepartment of Biomedical Chemistry, School of Science and Technology, Kwansei Gakuin University |
|---|
Research summary
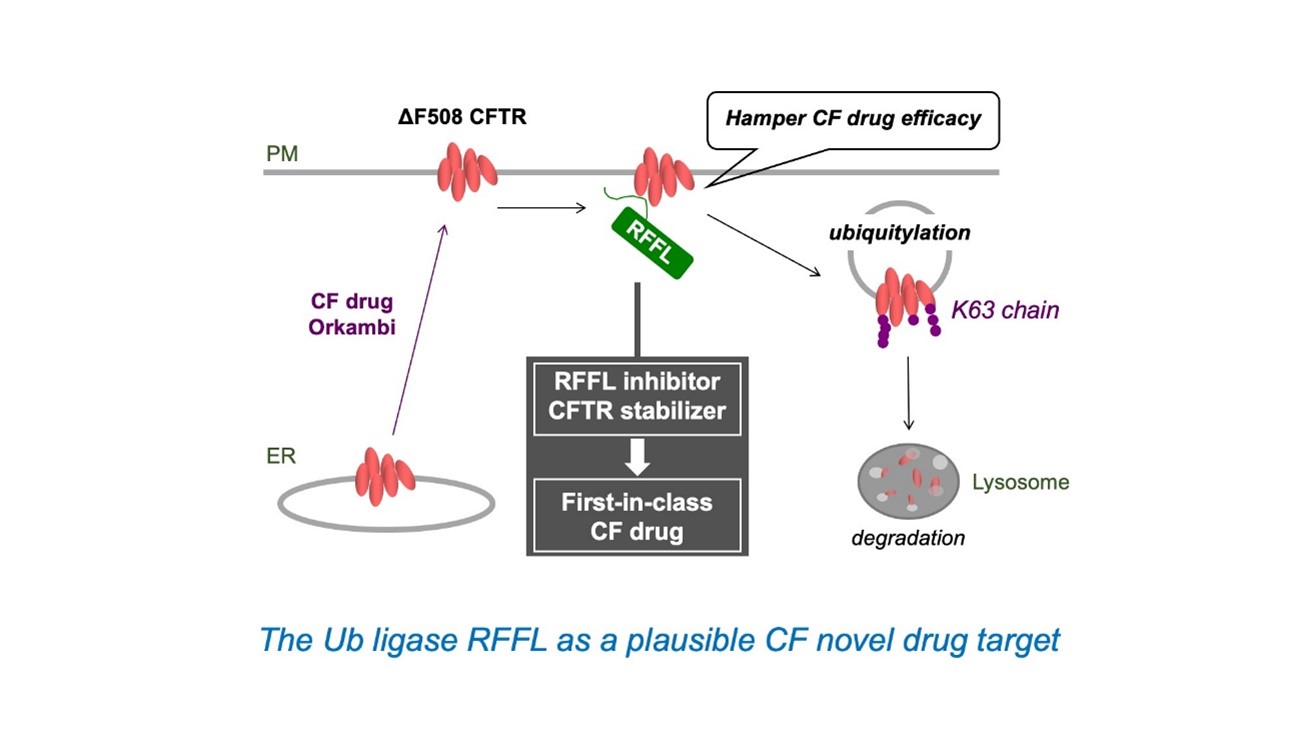
Cystic Fibrosis (CF) is a lethal monogenic disease that affects 80,000 patients worldwide (average lifespan ~40 years). The cause of CF is decreased expression of the CFTR protein at the plasma membrane (PM) caused by the gene mutation (ΔF508 mutation) in the CFTR chloride channel. Recently, a therapeutic agent for CF (Orkambi®), which promotes the PM expression and function of ΔF508 CFTR, has been approved. However, its clinical therapeutic benefit is limited. The development of a CFTR stabilizer that inhibits the CFTR elimination from the PM is necessary to establish the highly efficacious CF drug therapy. We have identified a ubiquitin ligase, RFFL, that promotes the ΔF508 CFTR elimination from the PM. RFFL knockdown (KD) enhances the efficacy of Orkambi® in cell culture models. RFFL directly interacts and selectively facilitates the unfolded CFTR ubiquitination. Because RFFL knockout (KO) mice exhibit no abnormal phenotype, RFFL-CFTR interaction (PPI) inhibitors may be CFTR stabilizers. We have also found that RFFL ubiquitinates the cytoplasmic region of the ΔF508 CFTR (ΔF508-NBD1) in cooperation with the E2 enzyme UbcH5c. Therefore, RFFL-UbcH5c PPI inhibition is expected to selectively inhibit the ubiquitination and degradation of ΔF508 CFTR from the PM and improve the efficacy of CF therapeutics.
In this project, we will elucidate the mechanism of CFTR-RFFL and UbcH5c-RFFL PPI and establish the approaches to regulate these PPI with artificial peptides (staple peptides, etc.). Moreover, we will clarify whether CFTR-RFFL and UbcH5c-RFFL PPI inhibitory peptides have a therapeutic effect on CF. Furthermore, we will develop RFFL degraders and other RFFL function inhibitors and evaluate their effect as CFTR stabilizers. We will also identify the ubiquitin code of abnormal CFTR, which functions as a signal for PM degradation and identify the ubiquitin decoder molecules that control the CFTR endocytosis and lysosomal degradation.
Publications
- Kamada Y, Fukuda R,*Okiyoneda T.
ELISA Based Protein Ubiquitylation Measurement.
Bio-protocol 9, e3430 (2019)
Former Publications
- Okiyoneda T, Veit G, Dekkers JF, Bagdany M, Soya N, Xu H, Roldan A, Verkman AS, Kurth M, Simon A, Hegedus T, Beekman JM, *Lukacs GL.
Mechanism-based corrector combination restores ΔF508-CFTR folding and function.
Nat. Chem. Biol. 9, 444-454 (2013)
PMID: 23666117 - Bagdany M, Veit G, Fukuda R, Avramescu RG, Okiyoneda T, Baaklini I, Singh J, Sovak G, Xu H, Apaja PM, Sattin S, Beitel LK, Roldan A, Colombo G, Balch W, Young JC, *Lukacs GL.
Chaperones rescue the energetic landscape of mutant CFTR at single molecule and in cell.
Nat. Commun. 8, 398 (2017)
PMID: 28855508 - *Okiyoneda T, Veit G, Sakai R, Aki M, Fujihara T, Higashi M, Susuki-Miyata S, Miyata M, Fukuda N, Yoshida A, Xu H, Apaja PM, Lukacs GL.
Chaperone-Independent Peripheral Quality Control of CFTR by RFFL E3 Ligase.
Dev. Cell 44, 694-708.e7 (2018)
PMID: 29503157 - Fukuda R, *Okiyoneda T.
Peripheral Protein Quality Control as a Novel Drug Target for CFTR Stabilizer.
Front. Pharmacol. 9, 1100 (2018)
PMID: 30319426 - Sakai R, Fukuda R, Unida S, Aki M, Ono Y, Endo A, Kusumi S, Koga D, Fukushima T, Komada M, *Okiyoneda T.
The integral function of the endocytic recycling compartment is regulated by RFFL-mediated ubiquitylation of Rab11 effectors.
J. Cell Sci. 132, jcs228007 (2019)
PMID: 30659120





