Tetsuo Narumi
Development of non-hydrolysable alkene-type ubiquitin bond isosteres for ubiquitin research
 |
Tetsuo Narumi, PhDShizuoka University, Graduate School of Integrated Science and Technology, Laboratory of Bioorganic Chemistry |
|---|
Research summary
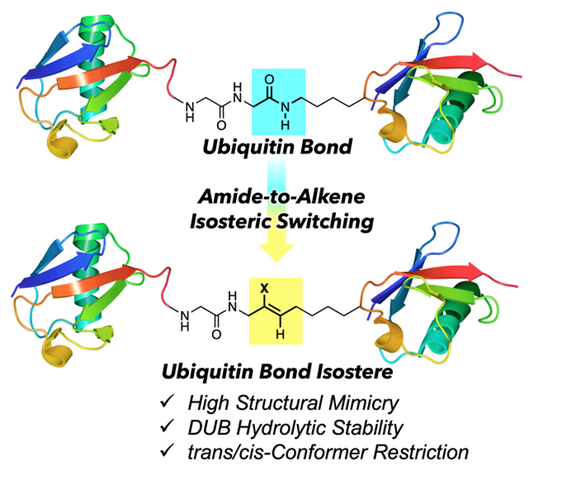
Ubiquitin (Ub) can form several types of ubiquitination linkages, including linear, mixed, or branched based on eight basic ubiquitination linkage types, whose linkage type and length of the ubiquitin chains determine the fate of the ubiquitinated proteins. To understand the details of ubiquitination signals, we will develop the Ub mimics, enabling the control of the spatial orientation of Ub chains based on the precisely controlled isosteric switching strategy. Mainly, we are interested in switching of isopeptide bond (Ub bond) with various alkenes such as (E)-methyl alkene and (Z)-chloroalkene to develop the alkene-type Ub bond isosteres. These studies provide the opportunity for future works, including the development of novel decoder molecules and deubiquitinase inhibitors.
Former Publications
- Narumi T, Hayashi R, Tomita K, Kobayashi K, Tanahara N, Ohno H, Naito T, Kodama E, Matsuoka M, *Oishi S, *Fujii S.
Synthesis and biological evaluation of selective CXCR4 antagonists containing alkene dipeptide isosteres.
Org. Biomol. Chem. 8, 616-621 (2009)
PMID: 20090978 - Narumi T, Takano H, Ohashi N, Suzuki A, Furuta T, *Tamamura H.
Isostere-Based Design of 8-Azacoumarin-Type Photolabile Protecting Groups: A Hydrophilicity Increasing Strategy for Coumarin-4-ylmethyls.
Org. Lett. 16, 1184-1187 (2014)
PMID: 24495035 - Kobayakawa T, *Narumi T, *Tamamura H.
Remote Stereoinduction in the Organocuprate-Mediated Allylic Alkylation of Allylic gem-Dichlorides: Highly Diastereoselective Synthesis of (Z)-Chloroalkene Dipeptide Isosteres.
Org. Lett. 17, 2302-2305 (2015)
PMID: 25950639 - Takano T, *Narumi T, Nomura W, Furuta T, *Tamamura H.
Utilization of the Heavy Atom Effect for the Development of a Photosensitive 8-Azacoumarin-Type Photolabile Protecting Group.
Org. Lett. 17, 5372-5375 (2015)
PMID: 26469518 - *Narumi T, Nishizawa T, Imai T, Kyan R, Taniguchi H, Sato K, Mase N.
Improvement of chemical stability of conjugated dienes by chlorine substitution.
Tetrahedron 74, 6257-6533 (2018)





