Daisuke Morito
Function and dysfunction of atypical ubiquitylation, and its artificial control
 |
Daisuke Morito, PhDShowa University School of Medicine, Department of Biochemistry |
|---|
Research summary
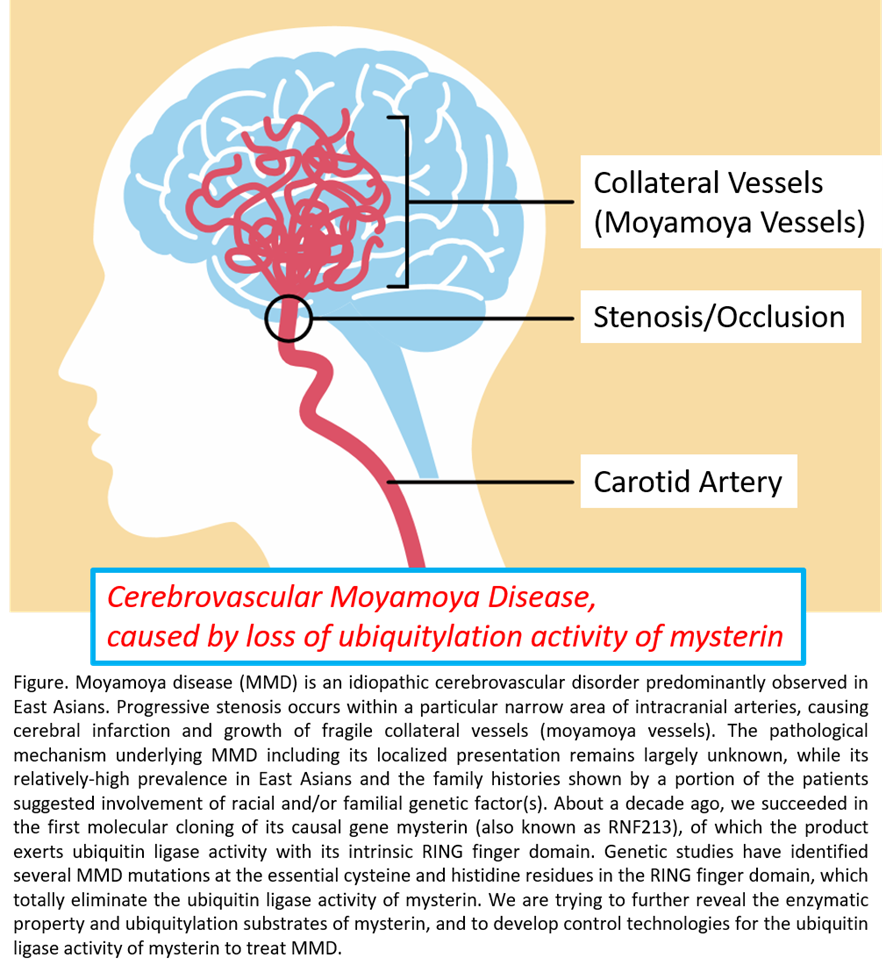
We isolated the full-length cDNA for a novel gene mysterin (also known as RNF213) about 10 years ago. It encodes a 591 kDa large intracellular enzyme which exerts locomotive ATPase (known as AAA+) and ubiquitin ligase activities. Genetic studies suggested that mutations in RING finger ubiquitin ligase domain of mysterin cause cerebrovascular moyamoya disease (MMD, characterized by severe stenosis/occlusion of cerebral arteries), while underlying pathogenic mechanism remains totally unknown. We identified that mysterin physiologically acts at lipid droplets (LDs), the ubiquitous organelles specialized for cellular lipid storage, with its ATPase and ubiquitin ligase activities. Mysterin stabilizes cellular fat storage selectively eliminating a major lipase ATGL from LDs. Intriguingly, the MMD mutations in mysterin’s RING finger domain largely impair such the action of mysterin; instead, the MMD mutations resulted in aggregate-like mislocalization of mysterin in cells, suggesting critical involvement of the ubiquitin ligase activity of mysterin in the pathogenesis of MMD. We are trying to identify mysterin’s ubiquitylation substrates and the modality of the ubiquitylation and unravel how mysterin with impaired ubiquitin ligase activity initiates/proceeds the pathogenic process of MMD focusing on atypical ubiquitylation activity that mysterin may exert. Besides, we try to develop the procedures to artificially restore/regulate mysterin’s ubiquitin ligase activity, through which we can approach the curative treatment of MMD that is currently absent.
Former Publications
- Morito D, Nishikawa K, Hoseki J, Kitamura A, Kotani Y, Kiso K, Kinjo M, Fujiyoshi Y, Nagata K.
Moyamoya disease-associated protein mysterin/RNF213 is a novel AAA+ ATPase, which dynamically changes its oligomeric state.
Sci. Rep. 4, 4442 (2014)
PMID: 24658080 - Kotani Y, *Morito D, Yamazaki S, Ogino K, Kawakami K, Takashima S, *Hirata H, *Nagata K.
Neuromuscular regulation in zebrafish by a large AAA+ ATPase/ubiquitin ligase, mysterin/RNF213.
Sci. Rep. 5, 16161 (2015)
PMID: 26530008 - Kotani Y, *Morito D, Sakata K, Ainuki S, Sugihara M, Hatta T, Iemura SI, Takashima S, Natsume T, Nagata K.
Alternative exon skipping biases substrate preference of the deubiquitylase USP15 for mysterin/RNF213, the moyamoya disease susceptibility factor.
Sci. Rep. 7, 44293 (2017)
PMID: 28276505 - Sugihara M, *Morito D, Ainuki S, Hirano Y, Ogino K, Kitamura A, Hirata H, Nagata K.
The AAA+ ATPase/ubiquitin ligase mysterin stabilizes cytoplasmic lipid droplets.
J. Cell Biol. 218, 949-960(2019)
PMID: 30705059