Atsushi Matsuzawa
Development of therapeutic strategies for various diseases by regulation of the cell death/survival balance using ubiquitin chemo-technologies
 |
Atsushi Matsuzawa, PhDLaboratory of Health Chemistry, Graduate School of Pharmaceutical Sciences, Tohoku University |
|---|
Research summary
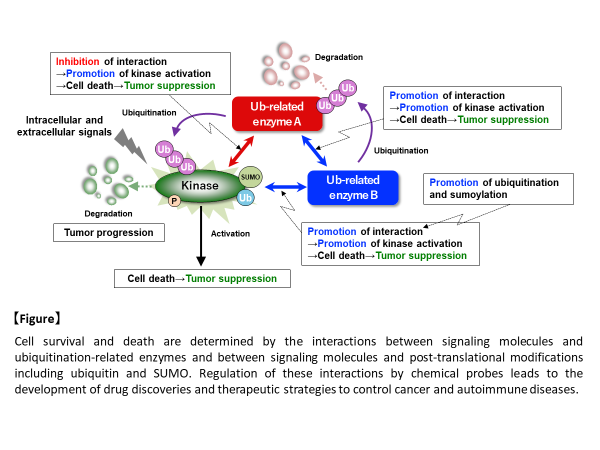
Cellular responses, such as cell viability, are determined by the balance of various signals, which are inputted from the inside and outside of the cells and regulate cell proliferation, differentiation, and death. Recently, we found that multiple ubiquitination-related enzymes clustered around signaling molecules regulating cell life and death, formed the complex, and controlled their activities each other to fine-tune the signal balance, which determines cell viability and induces appropriate cellular responses. When the signal balance is disrupted, abnormal signals are generated and induce excessive cell death and proliferation, leading to cancer and autoimmune diseases. We also found that post-translational modifications, such as ubiquitination and SUMOylation, are essential for the interaction between multiple ubiquitination-related enzymes in the complex, and therefore, the regulation of these modifications suppresses excessive cell death and proliferation and enables the treatment of various diseases, including cancer and autoimmune diseases, which are induced by the imbalance in the signals regulating cell life and death.
For example, a kinase that regulates cell viability forms a complex together with different ubiquitination-related enzymes, which control the activity of the kinase. One ubiquitination-related enzyme is directly involved in ubiquitination-dependent degradation of the kinase, and another ubiquitination-related enzyme contributes to ubiquitination-dependent degradation of the former ubiquitination-related enzyme, leading to indirect regulation of the kinase. Thus, such the complicated fine-tuning mechanism exists in the signals. The interaction between these molecules is found to require post-translational modifications, such as ubiquitination and SUMOylation. In other words, it is important to elucidate the mechanisms of the complex formation and the regulation of the signal balance determining cell life and death at the molecular level, because the interaction between signaling molecules and the recognition of ubiquitin and SUMO by signaling molecules are essential points (sites of action) for the regulation of the signal balance determining cell life and death. Therefore, we believe that these points can be artificially controled and fine-tuned by chemotechnology at the molecular level.
In this study, we would like to reveal that the controled signal balance of cellular responses, such as cell life and death, leads to the suppression of excessive cell death and proliferation, through appropriate regulation of the positive or negative interaction between ubiquitination-related modifications and signaling molecules, using chemotechnology, and to propose novel therapeutic strategies for cancer and immune diseases, which are different from conventional methods.
Publications
- Yokosawa T, Yamada M, Noguchi T, Suzuki S, Hirata Y, *Matsuzawa A.
Pro-caspase-3 protects cells from polymyxin B-induced cytotoxicity by preventing ROS accumulation.
J. Antibiotics 72, 848-852 (2019)
PMID: 31371783 - Sekiguchi Y, Yamada M, Noguchi T, Noomote C, Tsuchida M, Kudoh Y, Hirata Y, *Matsuzawa A.
The anti-cancer drug gefitinib accelerates Fas-mediated apoptosis by enhancing caspase-8 activation in cancer cells.
J. Toxicol. Sci. 44, 435-440 (2019)
PMID: 31168030 - Abe T, Shizu R, Sasaki T, Shimizu Y, Hosaka T, Kodama S, Matsuzawa A, Yoshinari K.
Functional Interaction between Pregnane X Receptor and Yes-Associated Protein in Xenobiotic-Dependent Liver Hypertrophy and Drug Metabolism.
J. Pharmacol. Exp. Ther. 371, 590-601 (2019)
PMID: 31533970 - Yamada M, Suzuki M, Noguchi T, Yokozawa T, Sekiguchi Y, Mutoh N, Toyama T, Hirata Y, Hwang GW, *Matsuzawa A.
The antibiotic cefotaxime works as both an activator of Nrf2 and an inducer of HSP70 in mammalian cells.
BPB Rep. 3, 16-21 (2020) - Hirata Y, Inoue A, Suzuki S, Takahashi M, Matsui R, Kono N, Noguchi T, *Matsuzawa A.
trans-Fatty acids facilitate DNA damage-induced apoptosis through the mitochondrial JNK-Sab-ROS positive feedback loop.
Sci. Rep. 10, 2743 (2020)
PMID: 32066809 - Tsuchida M, Yokosawa T, Noguchi T, Shimada T, Yamada M, Sekiguchi Y, Hirata Y, *Matsuzawa A.
Pro-apoptotic functions of TRAF2 in p53-mediated apoptosis induced by cisplatin.
J. Toxicol. Sci. 45, 219-226 (2020)
PMID: 32238696 - Luong NC, Abiko Y, Shibata T, Uchida K, Warabi E, Suzuki M, Noguchi T, Matsuzawa A, *Kumagai Y.
Redox cycling of 9,10-phenanthrenequinone activates epidermal growth factor receptor signaling through S-oxidation of protein tyrosine phosphatase 1B.
J. Toxicol. Sci. 45, 349-363 (2020)
PMID: 32493877 - Hirata Y, Nada Y, Yamada Y, Toyama T, Fukunaga K, Hwang GW, Noguchi T, *Matsuzawa A.
Elaidic acid potentiates extracellular ATP-induced apoptosis via the P2X7-ROS-ASK1-p38 axis in microglial cell lines.
Biol. Pharm. Bull. 43, 1562-1569 (2020)
PMID: 32999166 - Kudo T, Tominami K, Izumi S, Hayashi Y, Noguchi T, Matsuzawa A, Hong G, Nakai J.
Characterization of PC12 cell subclones with different sensitivities to programmedthermal stimulation.
Int. J. Mol. Sci., 21, 8356 (2020)
PMID: 33171774 - Suzuki M, Asai Y, Kagi T, Noguchi T, Yamada M, Hirata Y, *Matsuzawa A.
TAK1 Mediates ROS Generation Triggered by the Specific Cephalosporins through Noncanonical Mechanisms.
Int. J. Mol. Sci., 21, 9497 (2020)
PMID: 33327477 - Udagawa T, Seki M, Okuyama T, Adachi S, Natsume T, Noguchi T, Matsuzawa A, Inada T.
Failure to Degrade CAT-Tailed Proteins Disrupts Neuronal Morphogenesis and Cell Survival.
Cell Rep. 34, 108599 (2021)
PMID: 33406423 - Noguchi T, Sekiguchi Y, Kudoh Y, Naganuma R, Kagi T, Nishidate A, Maeda K, Ishi C, Toyama T, Hirata Y, Hwang GW, *Matsuzawa A.
Gefitinib initiates sterile inflammation by promoting IL-1β and HMGB1 release via two distinct mechanisms.
Cell Death Dis. 12, 49 (2021)
PMID: 33414419
Former Publications
- Matsuzawa A, Tseng PH, Vallabhapurapu S, Luo JL, Zhang W, Wang H, Vignali DA, Gallagher E, *Karin M.
Essential cytoplasmic translocation of a cytokine receptor-assembled signaling complex.
Science (Research Article) 321, 663-668 (2008)
PMID: 18635759 - Maruyama T, Araki T, Kawarazaki Y, Naguro I, Heynen S, Aza-Blanc P, Ronai Z, Matsuzawa A, *Ichijo H.
Roquin-2 promotes ubiquitin-mediated degradation of ASK1 to regulate stress responses.
Sci. Signal. 7, ra8 (2014)
PMID: 24448648 - *Matsuzawa A.
Thioredoxin and redox signaling: Roles of the thioredoxin system in control of cell fate.
Arch. Biochem. Biophys. 617, 101-105 (2017)
PMID: 27665998 - Hirata Y, Katagiri K, Nagaoka K, Morishita T, Kudoh Y, Hatta T, Naguro I, Kano K, Udagawa T, Natsume T, Aoki J, Inada T, Noguchi T, Ichijo H, *Matsuzawa A.
TRIM48 promotes ASK1 activation and cell death through ubiquitination-dependent degradation of the ASK1 negative regulator PRMT1.
Cell Rep. 21, 2447-2457 (2017)
PMID: 29186683 - Noguchi T, Suzuki M, Mutoh N, Hirata Y, Tsuchida M, Miyagawa S, Hwang G W, Aoki J, *Matsuzawa A.
Nuclear-accumulated SQSTM1/p62-based ALIS act as microdomains sensing cellular stresses and triggering oxidative stress-induced parthanatos.
Cell Death Dis. 9, 1193 (2018)
PMID: 30546061





