Hirotaka Takahashi
Development of small chemical compounds targeting deubiquitinating enzyme for regulation of the amounts of cellular proteins
 |
Hirotaka Takahashi, PhD.Division of Cell-free Sciences, Proteo-Science Center, Ehime University |
|---|
Research summary
Recently, chemical protein knockdown technologies like PROTAC and SNIPER that induce E3 ligase-dependent proteolysis of target proteins are rapidly developing. However, these technologies have been applied to only limited number of proteins because of the difficulty in development of the chemical compounds specifically binding to proteins of interest. In our research project, we focus on deubiquitinating enzymes (DUBs). Many DUBs remove degradative ubiquitin chains that conjugated to the target proteins, resulting in the protection of target proteins from 26S proteasomal degradation. If the activity of these DUBs could be controlled by small chemical compounds, it is highly possible to promote the degradation or conversely the stabilization of the proteins whose amounts are regulated by DUBs. However, only a few chemical compounds have been reported to regulate the activity of specific DUBs. The main reason for this is that the structure of the catalytic domain of the DUB is highly conserved among many DUBs, thus many of already reported DUB inhibitors nonspecifically inhibit a broad range of DUBs.
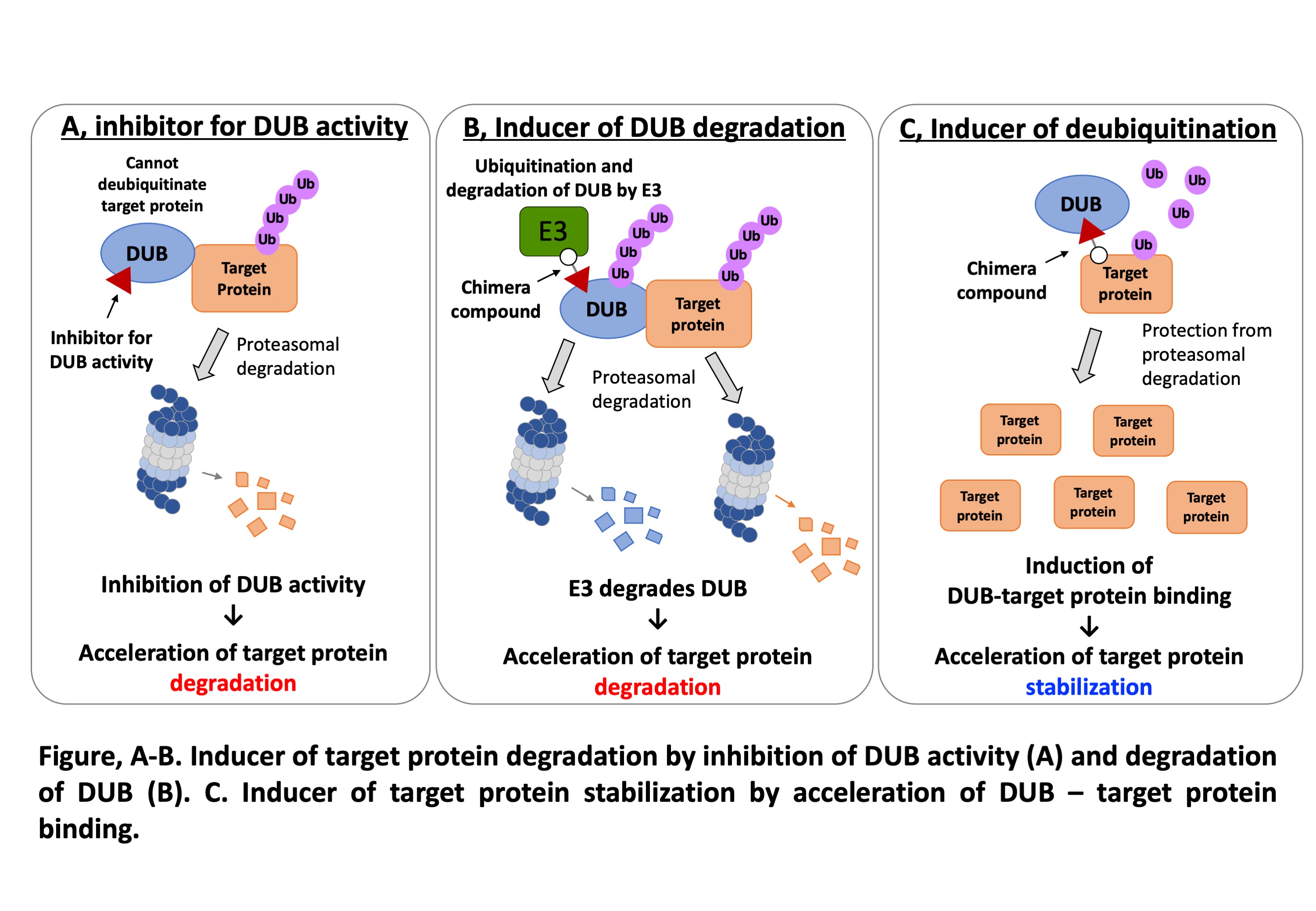
Based on a wheat cell-free protein expression system, which developed in our laboratory, an assay platform for high-throughput drug screening was established. Using this platform, we succeeded in development of a DUB inhibitor named Subquinocin that specifically inhibit the USP family DUBs. Currently, we are developing chemical compounds that inhibit only one or a few DUBs among the USP family by modification of the structure of Subquinocin. These specific USP inhibitors enable us to develop the DUB-dependent technology for protein knockdown (A and B in figure), and protein stabilization (C in figure). In this study, we selected USP15 as model DUB, because this DUB has been reported to be involved in development of cancer and neurodegenerative disease by stabilization of several cellular proteins including well-known oncoprotein, MDM2. Based on the already reported crystal structure of USP15, the specificity, binding affinity, and inhibitory effect of Subquinocin for individual USPs are modified using in silico docking model analysis and structural analysis. Then, obtained derivative compounds are used for the regulation of the amounts of cellular proteins in which USP15 and other USPs target. Using these chemical compounds as model, we aim to develop the technology for the chemical-based regulation of protein amount.
Publications
- Takaoka Y, Suzuki K, Nozawa A, Takahashi H, Sawasaki T, Ueda M.
Protein-protein interactions between jasmonate-related master regulator MYC and transcriptional mediator MED25 depend on a short binding domain.
J Biol Chem. 298, 101504 (2022)
PMID: 34929168 - Shioya R, Yomada K, Kido K, Takahashi H, Nozawa A, Miyakawa T, Kosako H, Sawasaki T.
A simple method for labeling proteins and antibodies with biotin using the proximity biotinylation enzyme TurboID.
Biochem Biophys Res Commun. 592, 54-59 (2022)
PMID: 35030423 - Oikawa D, Gi M, Kosako H, Shimizu K, Takahashi H, Shiota M, Hosomi S, Komakura K, Wanibuchi H, Tsuruta D, Sawasaki T, Tokunaga F.
OTUD1 deubiquitinase regulates NF-κB- and KEAP1-mediated inflammatory responses and reactive oxygen species-associated cell death pathways.
Cell Death Dis. 13, 694 (2022)
PMID: 35941131
Former Publications
- Takahashi H, Uematsu A, Yamanaka S, Imamura M, Nakajima T, Doi K, Yasuoka S, Takahashi C, Takeda H, *Sawasaki T.
Establishment of a Wheat Cell-Free Synthesized Protein Array Containing 250 Human and Mouse E3 Ubiquitin Ligases to Identify Novel Interaction between E3 Ligases and Substrate Proteins.
PLoS ONE 11, e0156718 (2016)
PMID: 27249653 - Uematsu A, Kido K, Takahashi H, Takahashi C, Yanagihara Y, Saeki N, Yoshida S, Maekawa M, Honda M, Kai T, Shimizu K, Higashiyama S, Imai Y, Tokunaga F, *Sawasaki T.
The E3 ubiquitin ligase MIB2 enhances inflammation by degrading the deubiquitinating enzyme CYLD.
J. Biol. Chem. 294, 14135–14148 (2019)
PMID: 31366726 - Yamanaka S, Sato Y, Oikawa D, Goto E, Fukai S, Tokunaga F, *Takahashi H, *Sawasaki T.
Subquinocin, a small molecule inhibitor of CYLD and USP-family deubiquitinating enzymes, promotes NF-κB signaling.
Biochem. Biophys. Res. Commun. 524, 1-7 (2020)
PMID: 31898971 - *Takahashi H, Yamanaka S, Kuwada S, Higaki K, Kido K, Sato Y, Fukai S, Tokunaga F, *Sawasaki T.
A Human DUB Protein Array for Clarification of Linkage Specificity of Polyubiquitin Chain and Application to Evaluation of Its Inhibitors.
Biomedicines 8, 152 (2020)
PMID: 32512835 - Yamanaka S, Murai H, Saito D, Abe G, Tokunaga E, Iwasaki T, Takahashi H, Takeda H, Suzuki T, Shibata N, Tamura K, Sawasaki T.
Thalidomide and its metabolite 5-hydroxythalidomide induce teratogenicity via the cereblon neo-substrate PLZF.
EMBO journal. 40, e105375 (2021)
PMID: 33470442