Tsukasa Okiyoneda
Development of agents regulating CFTR ubiquitination and application to CF drug discovery
 |
Tsukasa Okiyoneda, PhDDepartment of Biomedical Sciences, School of Biological and Environmental Sciences, Kwansei Gakuin University |
|---|
Research summary
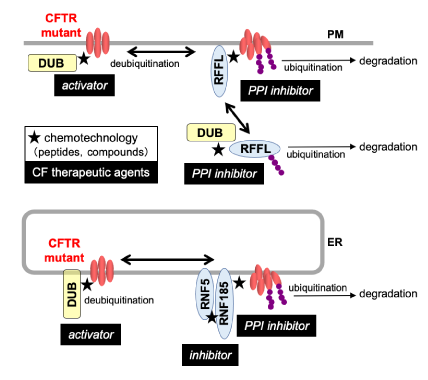
Cystic fibrosis (CF) is a lethal monogenic disease caused by the decreased expression of CFTR protein in the plasma membrane (PM) due to a gene mutation in the CFTR chloride channel. The most common mutant ∆F508-CFTR is misfolded and rapidly eliminated by protein quality control systems at the endoplasmic reticulum (ER) and PM by ubiquitination. Recently, CF therapeutic agents that promote the translocation of ∆F508-CFTR to the PM have been launched, but their therapeutic efforts are limited. Thus, agents that stabilize the mutant CFTR are expected to establish effective CF therapy. We have discovered a ubiquitin ligase (E3) RFFL, which promotes ∆F508-CFTR degradation from the PM, as a novel therapeutic target for CF (Dev Cell 2018). Moreover, we have identified RFFL ligands that could inhibit the RFFL-CFTR interaction (PPI) and the ∆F508-CFTR degradation. The double knockdown (KD) of E3 ligase RNF5 and RNF185 dramatically inhibits the ER-associated degradation (ERAD) of ∆F508-CFTR and enhances the effectiveness of CF therapeutic agents. We have identified compounds that bind to RNF5 and RNF185 by chemical array screening. In this project, we aim to develop the therapeutic agents by evaluating the effectiveness of these E3-related compounds on the ∆F508-CFTR in a primary culture of bronchial epithelial cells from CF donors (CF-HBE).
We have identified deubiquitinases (DUBs) that regulate ∆F508-CFTR PM expression by comprehensive phenotypic screening. These DUBs included those that bound to ∆F508-CFTR and those that bound to RFFL, a CFTR-related E3. In this study, we aim to develop a chemotechnology that controls CFTR-DUB and RFFL-DUB interactions to elucidate the function of the E3-DUB complex in the protein quality control mechanism.
Publications
- Taniguchi S, Ito Y, Kiritani H, Maruo A, Sakai R, Ono Y, Fukuda R, *Okiyoneda T.
The ubiquitin ligase RNF34 participates in the peripheral quality control of CFTR.
Front. Mol. Biosci. 9, 840649 (2022)
PMID: 35355508
Former Publications
- Okiyoneda T, Veit G, Dekkers JF, Bagdany M, Soya N, Xu H, Roldan A, Verkman AS, Kurth M, Simon A, Hegedus T, Beekman JM, *Lukacs GL.
Mechanism-based corrector combination restores ΔF508-CFTR folding and function.
Nat. Chem. Biol. 9, 444-454 (2013)
PMID: 23666117 - Bagdany M, Veit G, Fukuda R, Avramescu RG, Okiyoneda T, Baaklini I, Singh J, Sovak G, Xu H, Apaja PM, Sattin S, Beitel LK, Roldan A, Colombo G, Balch W, Young JC, *Lukacs GL.
Chaperones rescue the energetic landscape of mutant CFTR at single molecule and in cell.
Nat. Commun. 8, 398 (2017)
PMID: 28855508 - *Okiyoneda T, Veit G, Sakai R, Aki M, Fujihara T, Higashi M, Susuki-Miyata S, Miyata M, Fukuda N, Yoshida A, Xu H, Apaja PM, Lukacs GL.
Chaperone-Independent Peripheral Quality Control of CFTR by RFFL E3 Ligase.
Dev. Cell 44, 694-708.e7 (2018)
PMID: 29503157 - Sakai R, Fukuda R, Unida S, Aki M, Ono Y, Endo A, Kusumi S, Koga D, Fukushima T, Komada M, *Okiyoneda T.
The integral function of the endocytic recycling compartment is regulated by RFFL-mediated ubiquitylation of Rab11 effectors.
J. Cell Sci. 132, jcs228007 (2019)
PMID: 30659120 - Fukuda R, *Okiyoneda T.
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Ubiquitylation as a Novel Pharmaceutical Target for Cystic Fibrosis.
Pharmaceuticals (Basel). 13, 75 (2020) Review
PMID: 32331485





