Tetsuo Narumi
Development of chemo-technology for controlling the spatial orientation of ubiquitin chains
 |
Tetsuo Narumi, PhDShizuoka University, Graduate School of Integrated Science and Technology, Laboratory of Bioorganic Chemistry |
|---|
Research summary
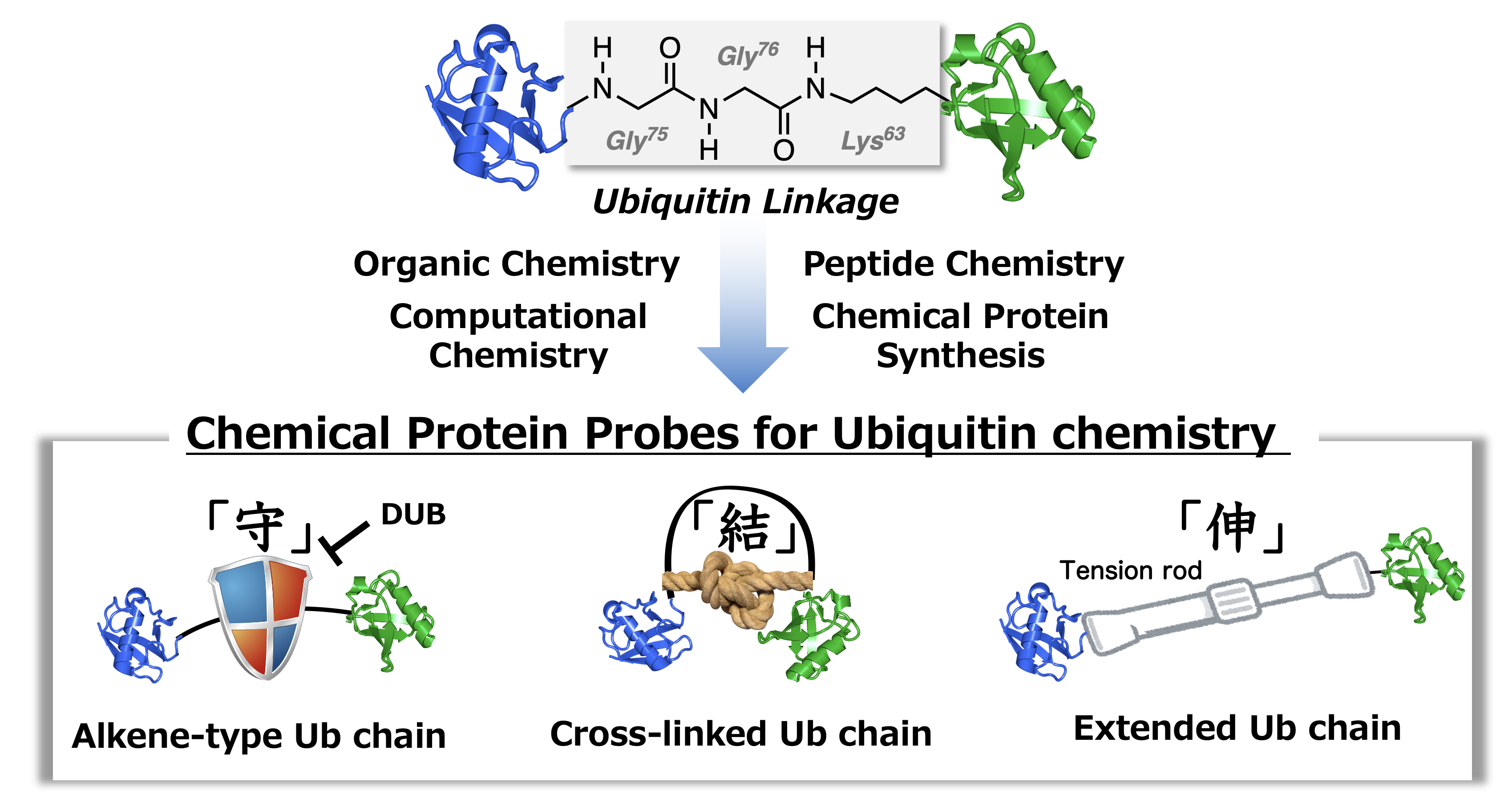
Chemical protein synthesis remains a challenge of great contemporary interest for many ubiquitin research. Exploration of the details of ubiquitination signals requires chemical probes of ubiquitin chains and ubiquitinated proteins with precisely controlled length and linkage types. Based on our experience gained from the peptidomimetic-based chemistry, backbone modifications of peptides including alkene dipeptide isosteres are suitable for controlling the spatial orientation of peptide chains.
Alkene dipeptide isosteres (ADIs) with the fixed cis/trans-conformation of the peptide bond enable the unequivocal exploration of molecular recognition based on the cis/trans-conformation of the peptide bond. The ADI methods can be easily combined with chemical protein synthesis to develop the chemical probe to control the spatial orientation of ubiquitin chains. To understand the details of ubiquitination signals, we will develop the mimics of ubiquitin chains and ubiquitinated proteins with chemically modified ubiquitination linkages that are important structural components of ubiquitin chains. These studies provide the opportunity for future works, including the development of novel decoder molecules and deubiquitinase inhibitors.
Publications
- Kodama Y, Takeo S, Fujimoto J, Sato K, Mase N, *Narumi T.
Synthesis and Structural Characterization of β-Turn Mimics Containing (Z)-Chloroalkene Dipeptide Isosteres.
J. Org. Chem. 87, 2167-2177 (2022)
PMID: 35179382
Former Publications
- Narumi T, Hayashi R, Tomita K, Kobayashi K, Tanahara N, Ohno H, Naito T, Kodama E, Matsuoka M, *Oishi S, *Fujii S.
Synthesis and biological evaluation of selective CXCR4 antagonists containing alkene dipeptide isosteres.
Org. Biomol. Chem. 8, 616-621 (2009)
PMID: 20090978 - Narumi T, Takano H, Ohashi N, Suzuki A, Furuta T, *Tamamura H.
Isostere-Based Design of 8-Azacoumarin-Type Photolabile Protecting Groups: A Hydrophilicity Increasing Strategy for Coumarin-4-ylmethyls.
Org. Lett. 16, 1184-1187 (2014)
PMID: 24495035 - Kobayakawa T, *Narumi T, *Tamamura H.
Remote Stereoinduction in the Organocuprate-Mediated Allylic Alkylation of Allylic gem-Dichlorides: Highly Diastereoselective Synthesis of (Z)-Chloroalkene Dipeptide Isosteres.
Org. Lett. 17, 2302-2305 (2015)
PMID: 25950639 - Takano T, *Narumi T, Nomura W, Furuta T, *Tamamura H.
Utilization of the Heavy Atom Effect for the Development of a Photosensitive 8-Azacoumarin-Type Photolabile Protecting Group.
Org. Lett. 17, 5372-5375 (2015)
PMID: 26469518 - Kyan R, Sato K, Mase N, *Narumi T.
Pendant Alkoxy Groups on N‐Aryl Substitutions Drive the Efficiency of Imidazolylidene Catalysts for Homoenolate Annulation from Enal and Aldehyde.
Angew. Chem. Int. Ed. 59, 19031-19036 (2020)
PMID: 32662539





