Daisuke Morito
Integrative Comprehension of Vasculopathies Originating from Ubiquitin Anomaly, and Drug Development
 |
Daisuke Morito, PhDShowa University School of Medicine, Department of Biochemistry |
|---|
Research summary
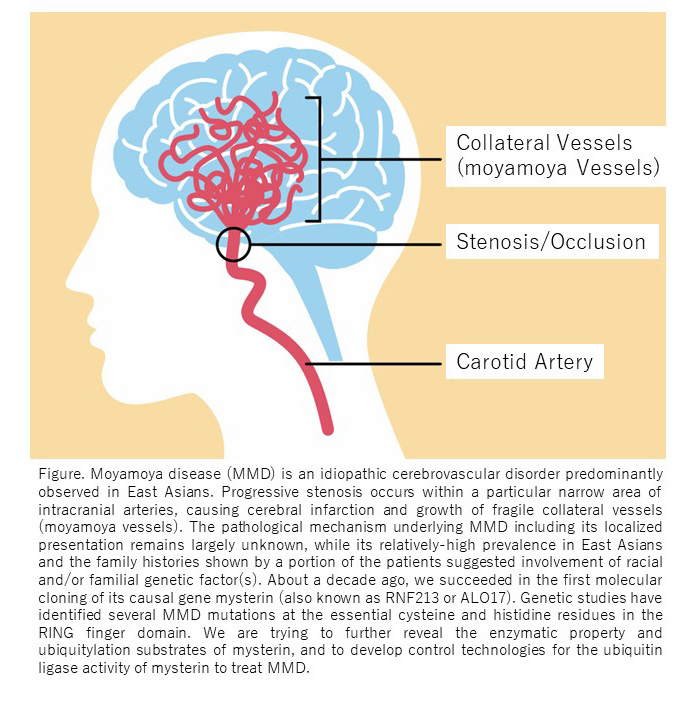
About a decade ago, I succeeded in the first molecular cloning of a new gene that we named mysterin (also known as RNF213 or ALO17) as a finishing touch of genetic exploration of cerebrovascular moyamoya disease (patent 5854423; PLOS ONE, 2011, cited 344 times). Mysterin gene encodes a unique large enzyme (about 600 kDa, the 21st largest protein within those encoded in the human genome) that harbors a dynein-like AAA+ ATPase core and a couple of ubiquitin ligase domains (RING finger and RZ finger). Patient mutations in the ubiquitin ligase domain of mysterin elevates the risk of arterial occlusion at the base of brain (i.e., moyamoya disease) to a varying degree, while its function at a molecular/cellular level has largely remained elusive within the subsequent years. We recently found mysterin is (surprisingly) located to the lipid droplets (LDs) which are the organelles specialized for lipid metabolism and exerts metabolic activity (J Cell Biol, 2019). The ATPase and ubiquitin ligase activities are both essential for such the function of mysterin. Recently, deeper structural-functional analyses suggest complex enzymatic mechanisms underlying its biological action, of which the whole picture is still largely elusive. In this project, we aim to reveal potential intra/inter-molecular enzymatic cooperation and its roles in the biological action of mysterin, particularly focusing on the ubiquitin ligase activity of mysterin and its significance in development of vascular anomalies including moyamoya disease.
Former Publications
- Morito D, Nishikawa K, Hoseki J, Kitamura A, Kotani Y, Kiso K, Kinjo M, Fujiyoshi Y, Nagata K.
Moyamoya disease-associated protein mysterin/RNF213 is a novel AAA+ ATPase, which dynamically changes its oligomeric state.
Sci. Rep. 4, 4442 (2014)
PMID: 24658080 - Kotani Y, *Morito D, Yamazaki S, Ogino K, Kawakami K, Takashima S, *Hirata H, *Nagata K.
Neuromuscular regulation in zebrafish by a large AAA+ ATPase/ubiquitin ligase, mysterin/RNF213.
Sci. Rep. 5, 16161 (2015)
PMID: 26530008 - Kotani Y, *Morito D, Sakata K, Ainuki S, Sugihara M, Hatta T, Iemura SI, Takashima S, Natsume T, Nagata K.
Alternative exon skipping biases substrate preference of the deubiquitylase USP15 for mysterin/RNF213, the moyamoya disease susceptibility factor.
Sci. Rep. 7, 44293 (2017)
PMID: 28276505 - Sugihara M, *Morito D, Ainuki S, Hirano Y, Ogino K, Kitamura A, Hirata H, Nagata K.
The AAA+ ATPase/ubiquitin ligase mysterin stabilizes cytoplasmic lipid droplets.
J. Cell Biol. 218, 949-960(2019)
PMID: 30705059





