Taku Kitanosono
Ubiquitin-selective recognition and labeling based on macromolecular self-assembly
 |
Taku Kitanosono, PhDThe University of Tokyo, Synthetic Organic Chemistry Laboratory |
|---|
Research summary
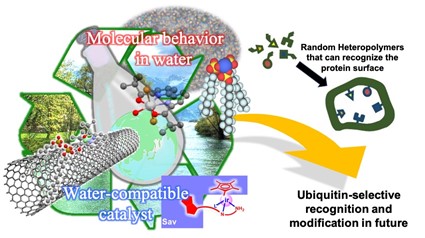
Recent advances in molecular recognition and organic synthesis has induced intensified competition for functionalizing and labeling of natural proteins by chemical modification all over the world. The promising transformations are molded by the need for biologically ambient conditions including optimal pH, temperature, low concentration, and aqueous environments, so as not to disrupt protein architecture or function. Hence, most approaches rely upon small molecules-based strategy using reactive chemicals together with trace amount of water-miscible organic solvents to mimic the reactions performed in organic solvents. Small molecules-based strategy is, however, intrinsically not suitable for tight and specific recognition of proteins leading to chemical modification. In particular, because ubiquitinated proteins readily undergo proteasomal degradation or deubiquitination, efficient detection of ubiquitin chains in vivo is one of the most challenging tasks. To find de novo molecules capable of tightly and specifically binding ubiquitin chains, our approach relies upon screening chemical libraries of synthetic random copolymers bearing multiple functionalities that would dovetail into characteristic chemical patterns on protein surfaces. We believe that this approach would complement conventional small molecules-based approaches and open new opportunities for specific labeling of ubiquitin chains without degradation or deubiquitination in compartmentalized environments. While developing reactions and catalysis in water, our group has been exploring unique organic chemistry in water that cannot be found in organic solvents. We believe that our knowledge on molecular behaviors, molecular recognition, and chemical reactivities in aqueous environments would contribute to offering new chemo-technology.
Publications
- *Kitanosono T, Hisada T, Yamashita Y, Kobayashi S.
Hydrogen-Bonding-Assisted Cationic Aqua Palladium(II) Complex Enables Highly Efficient Asymmetric Reactions in Water.
Angew. Chem. Int. Ed.60, 3407-3411 (2021)
PMID: 33124701
Former Publications
- Kitanosono T, Zhu L, Liu C, Xu P, *Kobayashi S.
An Insoluble Copper(II) Acetylacetonate−Chiral Bipyridine Complex that Catalyzes Asymmetric Silyl Conjugate Addition in Water.
J. Am. Chem. Soc. 137, 15422-15425 (2015)
PMID: 26646601 - Liu Z, Lebrun V, Kitanosono T, Mallin H, Köhler V, Häussinger D, Hilvert D, Kobayashi S, Ward TR.
Upregulation of an Artificial Zymogen by Proteolysis.
Angew. Chem. Int. Ed. 55, 11587-11590 (2016)
PMID: 27529471 - Kitanosono T, Miyo M, *Kobayashi S.
Surfactant-aided chiral palladium(II) catalysis exerted exclusively in water for the C–H functionalization of indoles.
ACS Sustainable Chem. Eng. 4, 6101-6106 (2016) - Kitanosono T, Masuda K, Xu P, *Kobayashi S.
Catalytic Organic Reactions in Water toward Sustainable Society.
Chem. Rev. 118, 679-746 (2018)
PMID: 29218984 - Kitanosono T, Xu P, *Kobayashi S.
Chiral Lewis acids integrated with single-walled carbon nanotubes for asymmetric catalysis in water.
Science 362, 311-315 (2018)
PMID: 30337405





