Yukihiro Itoh
Development of chemical probes for ubiquitinated proteins and their linkage patterns
 |
Yukihiro Itoh, PhDDepartment of Complex Molecular Chemistry The Institute of Scientific and Industrial Research, Osaka University |
|---|
Research summary
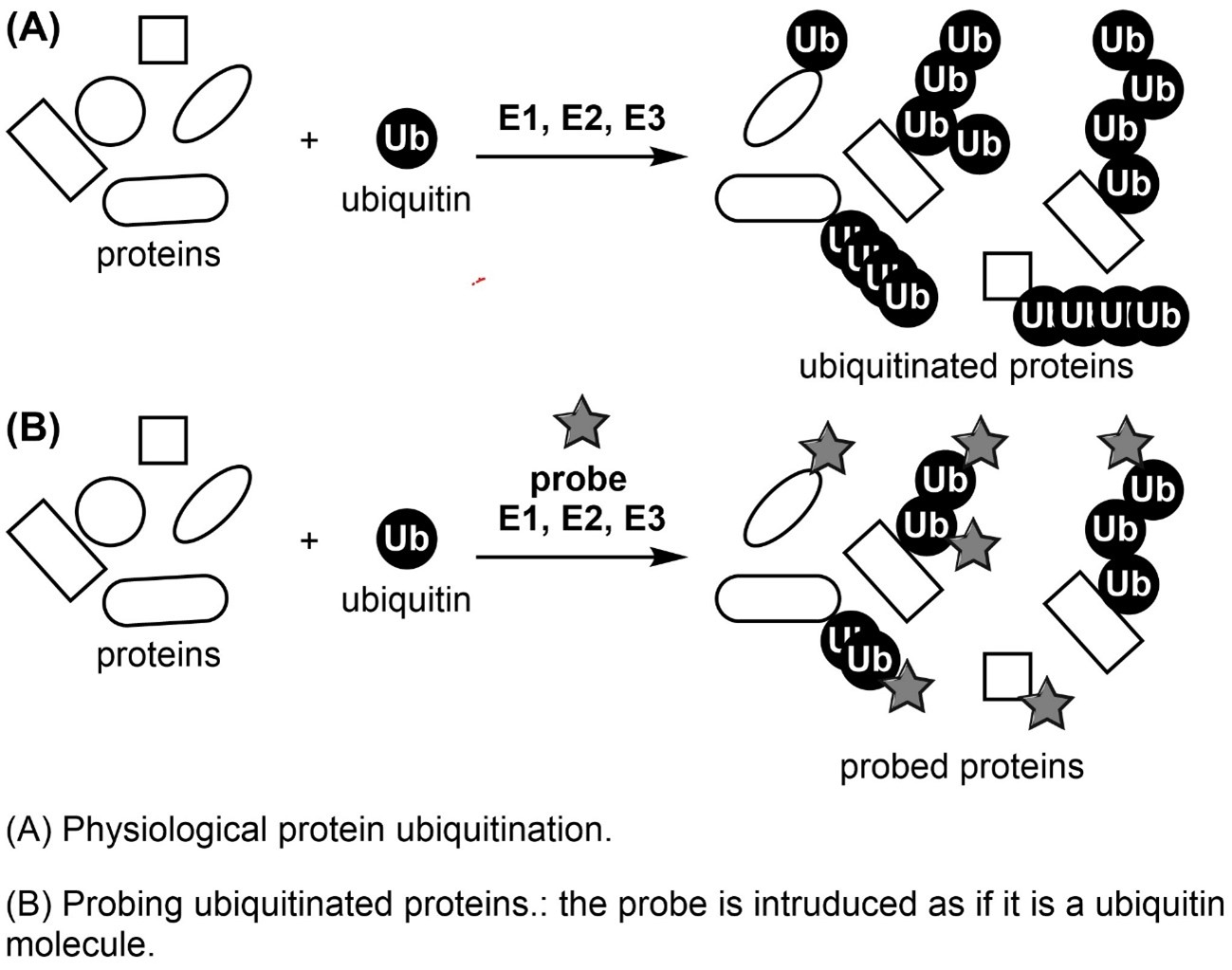
Protein ubiquitination plays important roles in several biological processes such as protein degradation, regulation of cell cycle, and cell differentiation. These biological phenomena are thought to be controlled by complicated ubiquitination patterns. Mono-ubiquitination and eight poly-ubiquitin chains are common protein ubiquitination patterns. Other ubiquitination patterns include mixed polyubiquitin chains, in which different types of ubiquitin chains are conjugated, and multiple branched ubiquitin chains, in which more than two ubiquitin chains are added to one ubiquitin. The complicated ubiquitination system is considered to precisely control the several biological processes. There is a need to better understand the relationship between protein ubiquitination patterns and the biological processes involved. However, it is difficult to detect or quantify ubiquitinated proteins and their linkage patterns in cells using current technologies, and they seldom reveal the relationship with the biological processes involved. Thus, we need to establish methodologies to detect ubiquitination patterns in cells more easily and efficiently.
In our previous study, we have developed some small molecules from organic chemistry that control protein ubiquitination. In this study, based on these findings, we try to develop small molecular probes to detect ubiquitinated proteins and their linkage patterns in the cell. In addition, we aim to establish a novel methodology to better understand cellular ubiquitination by these chemical probes.
Publications
- Iida T, *Itoh Y, Takahashi Y, Yamashita Y, Kurohara T, Miyake Y, Oba M, Suzuki T.
Design, Synthesis, and Biological Evaluation of Lysine Demethylase 5 C Degraders.
ChemMedChem AOP (2021)
PMID: 33470543
Former Publications
- Itoh Y, Ishikawa M, Naito M, *Hashimoto Y.
Protein knockdown using methyl bestatin-ligand hybrid molecules: design and synthesis of inducers of ubiquitination-mediated degradation of cellular retinoic acid-binding proteins.
J. Am. Chem. Soc. 132, 5820-5826 (2010)
PMID: 20369832 - Itoh Y, Ishikawa M, Kitaguchi R, Sato S, Naito M, *Hashimoto Y.
Development of target protein-selective degradation inducer for protein knockdown.
Bioorg. Med. Chem. 19, 3229-3241 (2011)
PMID: 21515062 - Itoh Y, Ishikawa M, Kitaguchi R, Okuhira K, Naito M, *Hashimoto Y.
Double protein knockdown of cIAP1 and CRABP-II using a hybrid molecule consisting of ATRA and IAPs antagonist.
Bioorg. Med. Chem. Lett. 22, 4453-4457 (2012)
PMID: 22658364 - *Itoh Y, Suzuki M.
Design, synthesis, and biological evaluation of novel ubiquitin-activating enzyme inhibitors.
Bioorg. Med. Chem. Lett. 28, 2723-2727 (2018)
PMID: 29548576 - *Itoh Y.
Chemical Protein Degradation Approach and its Application to Epigenetic Targets.
Chem. Rec. 9, 1681-1700 (2018)
PMID: 29893461





