Toshiaki Fukushima
The mechanism of action of the deubiquitinating enzyme USP8 revealed by chemo-technologies
 |
Toshiaki Fukushima, PhDTokyo Institute of Technology, Institute of Innovative Research, Cell Biology Center, Komada Laboratory |
|---|
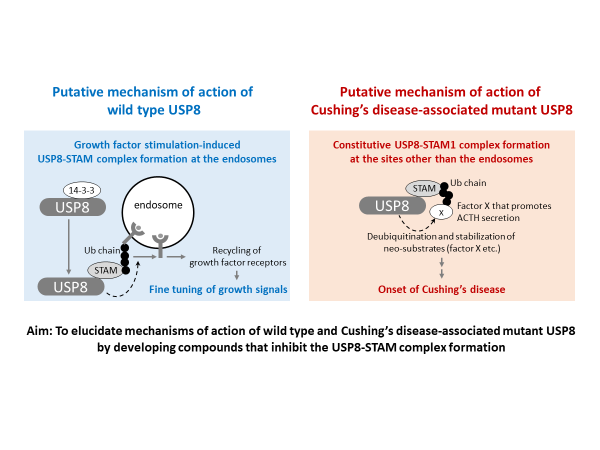
Research summary
We have reported that ubiquitin-specific protease 8 (USP8) deubiquitinates several endosomal membrane receptors, including the epidermal growth factor receptor, thereby promoting their recycling to the plasma membrane. Thus, USP8 has been hypothesized to potentiate growth factor signaling. However, the role of USP8 remains controversial since it is also required for the maintenance of endocytic machinery components, and its genetic depletion eventually leads to endocytic pathway impairment and cell death. Furthermore, the molecular mechanisms by which USP8 preferentially deubiquitinates specific endosomal proteins remain unclear.
USP8 harbors a 14-3-3-binding motif and a STAM-binding motif in close proximity to each other. STAM, a ubiquitin-binding protein, can promote the USP8-induced deubiquitination of target proteins. We recently found that 14-3-3 and STAM competitively bound to USP8. We hypothesize that 14-3-3 and STAM may regulate the USP8 enzymatic activity and intracellular localization, which enables an appropriate recycling of the specific endosomal proteins to the plasma membrane.
In contrast, we have previously reported that USP8 mutations cause the Cushing’s disease, an intractable endocrine disease. The patients oversecrete the adrenocorticotropic hormone (ACTH) followed by a hypercortisolism, leading to the onset of symptoms including a moon face and obesity, and a high risk of hypertension, diabetes, and immune-related disorders. We found amino acid substitutions and deletions in the 14-3-3-binding motif of USP8 in nearly 50% of the patients. We demonstrated that corticotrophs expressing the mutant USP8 excessively secreted ACTH.
Our subsequent study revealed that the mutant USP8 could not bind to 14-3-3 but effectively bound to STAM. Our preliminary experiments suggest that the Cushing’s disease-associated mutation causes the constitutive formation of the USP8–STAM complexes, which seem to be localized to the cellular compartments other than the endosome, where they may deubiquitinate the neo-substrates. Possibly through these mechanisms, the abnormal USP8–STAM complexes may cause the Cushing’s disease onset.
In this study, we aim to develop compounds that inhibit the USP8–STAM complex formation. We will manipulate the complex formation using these compounds, and investigate the mechanism of action of the wild-type and the Cushing’s disease-associated mutant USP8.
Publications
- Naito S, *Fukushima T, Endo A, Denda K, *Komada M.
Nik-related kinase is targeted for proteasomal degradation by the chaperone-dependent ubiquitin ligase CHIP.
FEBS Lett. 594, 1778-1786 (2020)
PMID: 32162334
Former Publications
- Fukushima T, Yoshihara H, Furuta H, Hakuno F, Luan J, Duan C, Saeki Y, Tanaka K, Iemura SI, Natsume T, Chida K, Nakatsu Y, Kamata H, Asano T, *Takahashi SI.
Nedd4-induced monoubiquitination of IRS-2 enhances IGF signaling and mitogenic activity.
Nat. Commun. 6, 6780 (2015)
PMID: 25879670 - *Girnita L, Takahashi SI, Crudden C, Fukushima T, Worrall C, Furuta H, Yoshihara H, Hakuno F, Girnita A.
Chapter Seven - When Phosphorylation Encounters Ubiquitination: A Balanced Perspective on IGF-1R Signaling.
Prog. Mol. Biol. Transl. Sci. 141, 277-311 (2016)
PMID: 27378760 - Furuta H, Yoshihara H, Fukushima T, Yoneyama Y, Ito A, Worrall C, Girnita A, Girnita L, Yoshida M, Asano T, Komada M, Kataoka N, Chida K, Hakuno F, *Takahashi SI.
IRS-2 deubiquitination by USP9X maintains anchorage-independent cell growth via Erk1/2 activation in prostate carcinoma cell line.
Oncotarget. 9, 33871-33883 (2018)
PMID: 30338032 - Xie X, Matsumoto S, Endo A, *Fukushima T, Kawahara H, Saeki Y, *Komada M.
Deubiquitylases USP5 and USP13 are recruited to and regulate heat-induced stress granules through their deubiquitylating activities.
J. Cell Sci. 131, jcs210856 (2018)
PMID: 29567855 - Kawaguchi K, Endo A, *Fukushima T, Madoka Y, Tanaka T, *Komada M.
Ubiquitin-specific protease 8 deubiquitinates Sec31A and decreases large COPII carriers and collagen IV secretion.
Biochem. Biophys. Res. Commun. 499, 635-641 (2018)
PMID: 29604273 - Sakai R, Fukuda R, Unida S, Aki M, Ono Y, Endo A, Kusumi S, Koga D, Fukushima T, Komada M, *Okiyoneda T.
The integral function of the endocytic recycling compartment is regulated by RFFL-mediated ubiquitylation of Rab11 effectors.
J. Cell Sci. 132, jcs228007 (2019)
PMID: 30659120





