Jun Arii
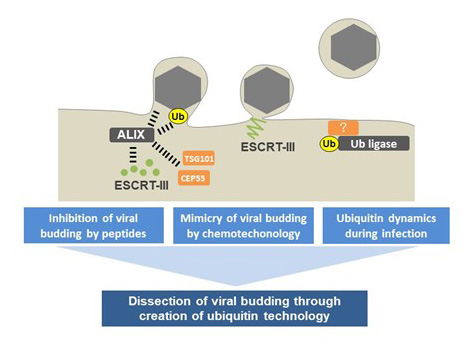
Dissection of viral budding through creation of ubiquitin technology
 |
Jun Arii, DVM, PhDDivision of Clinical Virology, Center for Infectious diseases, Kobe University Graduate School of Medicine |
|---|
Research summary
The endosomal sorting complexes required for transport (ESCRT)-III system is highly associated with ubiquitination and mediates reverse-topology membrane scission. Budding of most enveloped viruses from cellular membranes depends on ESCRT-III system and its adaptor proteins. However, it appears that the mammalian ESCRT pathway functions in a series of key cellular processes, including formation of intraluminal/extracellular vesicles, the abscission stage of cytokinesis, plasma membrane repair, and nuclear membrane fusion. Thus, it is not known whether ubiquitination of viral protein directly mediates viral budding.
Herpesviruses are common pathogenic agents causing a variety of diseases, such as mucocutaneous and skin diseases, keratitis, tumor, and encephalitis, in humans. They also establish life-long latent infections in the host, and almost all humans are already infected with them. Although antivirals have been developed against herpesviruses, they are incapable of eradicating the diseases caused by these viruses. Herpesviruses encode both ubiquitin ligase and deubiquitinase that play important roles in the evasion from host antiviral response, suggesting that study on the interaction between ubiquitination and herpesviruses is required to control their infection. We are trying to investigate importance of ubiquitination on viral proteins following herpesvirus infection, which induces budding from inner nuclear membrane and cytoplasmic membrane. The data from our study may help to clarify the molecular mechanism of viral budding that could serve as a potential therapeutic target for viral infections.
Publications
- Arii J, Takeshima K, Maruzuru Y, Koyanagi N, Kato A, Kawaguchi Y.
Roles of the interhexamer Contact Site for Hexagonal Lattice Formation of the Herpes Simplex Virus 1 Nuclear Egress Complex in Viral Primary Envelopment and Replication.
J. Virol. 93, e00498-19 (2019)
PMID: 31043535 - Takeshima K, Arii J (contributed equally), Maruzuru Y, Koyanagi N, Kato A, Kawaguchi Y.
Identification of the Capsid Binding Site in the Herpes Simplex Virus 1 Nuclear Egress Complex and Its Role in Viral Primary Envelopment and Replication.
J. Virol. 93, e01290-19 (2019)
PMID: 31391274 - Arii J, Maeda F, Maruzuru Y, Koyanagi N, Kato A, Mori Y, Kawaguchi Y.
ESCRT-III controls nuclear envelope deformation induced by progerin.
Sci. Rep. 10, 18877 (2020)
PMID: 33139753 - Arii J, Fukui A, Shimanaka Y, Kono N, Arai H, Maruzuru Y, Koyanagi N, Kato A, Mori Y, Y Kawaguchi.
Role of phosphatidyl-ethanolamine biosynthesis in herpes simplex virus 1 infected cells on progeny virus morphogenesis in the cytoplasm and on viral pathogenicity in vivo.
J. Virol. 94, e01572-20 (2020)
PMID: 32999028 - *Watanabe M, *Arii J, Takeshima K, Fukui A, Shimojima M, Kozuka-Hata H, Oyama M, Minamitani T, Yasui T, Kubota Y, Takekawa M, Kosugi I, Maruzuru Y, Koyanagi N, Kato A, Mori Y, Kawaguchi Y.
Prohibitin-1 contributes to the cell-to-cell transmission of herpes simplex virus 1 via the MAPK/ERK signaling pathway.
J. Virol. 95, e01413-20 (* contributed equally) (2021)
PMID: 33177205
Former Publications
- Arii J, Goto H, Suenaga T, Oyama M, Kozuka-Hata H, Imai T, Minowa A, Akashi H, Arase H, Kawaoka Y, *Kawaguchi Y.
Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus 1.
Nature 467, 859-862 (2010)
PMID: 20944748 - Arii J, Hirohata Y, Kato A, *Kawaguchi Y.
Nonmuscle Myosin Heavy Chain IIB Mediates Herpes Simplex Virus 1 Entry.
J. Virol. 89, 1879-1888 (2015)
PMID: 25428876 - Arii J, Shindo K, Koyanagi N, Kato A, *Kawaguchi Y.
Multiple Roles of the Cytoplasmic Domain of Herpes Simplex Virus 1 Envelope Glycoprotein D in Infected Cells.
J. Virol. 90, 10170-10181 (2016)
PMID: 27581980 - Arii J, Watanabe M, Maeda F, Tokai-Nishizumi N, Chihara T, Miura M, Maruzuru Y, Koyanagi N, Kato A, *Kawaguchi Y.
ESCRT-III mediates budding across the inner nuclear membrane and regulates its integrity.
Nat. Commun. 9, 3379 (2018)
PMID: 30139939 - *Arii J, Kawaguchi Y.
The Role of HSV Glycoproteins in Mediating Cell Entry.
Adv. Exp. Med. Biol. 1045, 3-21 (2018)
PMID: 29896660





