Research Group
TeamResearch Group
Research group A02: Development of ubiquitin chemo-technologies
Development of chemical protein knockdown technologies and their application to manipulate cellular functions
Mikihiko Naito, PhDGraduate School of Pharmaceutical Sciences, The University of Tokyo |
|
Yosuke Demizu, PhDDivision of Organic Chemistry, National Institute of Health Sciences |
|
Minoru Ishikawa, PhDGraduate School of Life Sciences, Tohoku University |
|
Takumi Ito, PhDDepartment of Chemical Biology, Tokyo Medical University |
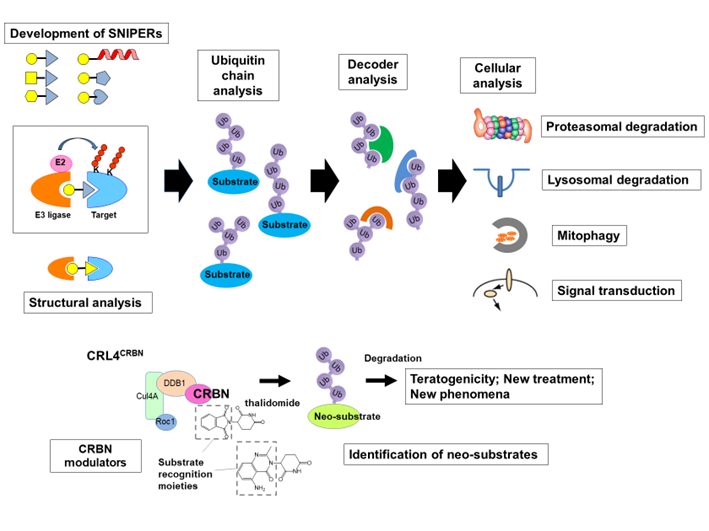
We have established chemical protein knockdown technologies that utilize compounds such as SNIPERs (Specific and Nongenetic IAP-dependent Protein Erasers) and cereblon (CRBN) modulators
(also known as IMiDs immunomodulators) to induce ubiquitylation and proteasomal degradation of target proteins in cells. SNIPER is a chimeric compound consisting of a ligand (IAP antagonist) that binds to an IAP and a ligand (molecular warhead) that binds to a target protein, which induces ubiquitylation by recruiting an IAP ubiquitin ligase to the target protein. It is a highly versatile technology that can target and degrade various target proteins by replacing molecular warheads. The CRBN modulator, on the other hand, is a compound that binds to CRBN, the substrate recognition molecule of CRL4CRBN ubiquitin ligase, and modulates its substrate specificity. These compounds have recently been shown to be able to induce various cellular responses involving ubiquitin, including not only degradation but also internalization of receptors on cell surface.
In this study, we are challenging to elucidate the principle of the ubiquitin code by using chemical protein knockdown technologies to ubiquitylate various target proteins and analyze the cellular responses, which includes the following subjects.
1. Development and expansion of SNIPER technology
2. Analysis of ubiquitin coding mechanism using SNIPER and other compounds
3. Development of the CRBN modulators and the mechanism of teratogenicity and therapeutic effect
4.Structural analysis of E3 ligase and neo-substrate complexes formed by SNIPER
5. Development of mitophagy induction technology
Publications
- Nishiyama Y, Fujii S, Makishima M, Hashimoto Y, Ishikawa M.
Efficient lead finding, activity enhancement and preliminary selectivity control of nuclear receptor ligands bearing a phenanthridinone skeleton.
Int. J. Mol. Sci. 19, 2090 (2018)
PMID: 30021999 - Shibata N, Shimokawa K, Nagai K, Ohoka N, Hattori T, Miyamoto N, Ujikawa O, Sameshima T, Nara H, Cho N, Naito M.
Pharmacological difference between degrader and inhibitor against oncogenic BCR-ABL kinase.
Sci. Rep. 8, 13549 (2018)
PMID: 30202081 - Shioi R, Toyota Y, Noguchi-Yachide T, Ishikawa M,Yamaguchi T, Makishima M, Hashimoto Y, Ohgane K.
Unexpected emergence of luciferase-inhibitory activity during structural development study of phenyloxadiazole-based PPAR ligands.
Heterocycles 97, 854-864 (2018) - Misawa T, Goto C, Shibata N, Hirano M, Kikuchi Y, Naito M, Demizu Y.
Rational design of novel amphipathic antimicrobial peptides focused on distribution of cationic amino acid residues.
MedChemComm 10, 896-900 (2019)
PMID: 31303986 - Ohoka N, Ujikawa O, Shimokawa K, Sameshima T, Shibata N, Hattori T, Nara H, Cho N, Naito M.
Different Degradation Mechanisms of Inhibitor of Apoptosis Proteins (IAPs) by the Specific and Nongenetic IAP-Dependent Protein Eraser (SNIPER).
Chem. Pharm. Bull. 67, 203-209 (2019)
PMID: 30369550 - Ando H, Sato T, Ito T, Yamamoto J, Sakamoto S, Nitta N, Asatsuma-Okumura T, Shimizu N, Mizushima R, Aoki I, Imai T, Yamaguchi Y, Berk AJ, Handa H.
Cereblon Control of Zebrafish Brain Size by Regulation of Neural Stem Cell Proliferation.
iScience 15, 95-108 (2019)
PMID: 31055217 - Misawa T, Ohoka N, Oba M, Yamashita H, Tanaka M, Naito M, Demizu Y.
Development of 2-aminoisobutyric acid (Aib)-rich cell-penetrating foldamers for efficient siRNA delivery.
Chem. Commun. 55, 7792-7795 (2019)
PMID: 31210205 - Asatsuma-Okumura T, Ando H, De Simone M, Yamamoto J, Sato T, Shimizu N, Asakawa K, Yamaguchi Y, Ito T, Guerrini L, Handa H.
p63 is a cereblon substrate involved in thalidomide teratogenicity.
Nat. Chem. Biol. 15, 1077-1084 (2019)
PMID: 31591562 - Ohoka N, Tsuji G, Shoda T, Fujisato T, Kurihara M, Demizu Y, Naito M.
Development of Small Molecule Chimeras That Recruit AhR E3 Ligase to Target Proteins.
ACS Chem. Biol. 14, 2822-2832 (2019)
PMID: 31580635 - Goto C, Hirano M, Hayashi K, Kikuchi Y, Hara-Kudo Y, Misawa T, Demizu Y.
Development of amphipathic antimicrobial peptide foldamers based on Magainin 2 sequence.
ChemMedChem 14, 1911-1916 (2019)
PMID: 31667994 - Yamashita H, Tomoshige S, Nomura S, Ohgane K, Hashimoto Y, Ishikawa M.
Application of Protein Knockdown Strategy Targeting β-Sheet Structure to Multiple Disease-associated Polyglutamine Proteins.
Bioorg. Med. Chem. 28, 115175 (2020)
PMID: 31767406 - Tateno S, Iida M, Fujii S, Suwa T, Katayama M, Tokuyama H, Yamamoto J, Ito T, Sakamoto S, Handa H, Yamaguchi Y.
Genome-wide screening reveals a role for subcellular localization of CRBN in the anti-myeloma activity of pomalidomide.
Sci. Rep. 10, 4012 (2020)
PMID: 32132601 - *Shoda T, Ohoka N, Tsuji G, Fujisato T, Inoue H, Demizu Y, Naito M, Kurihara M.
Targeted protein degradation by chimeric compounds using hydrophobic E3 ligands and adamantane moiety.
Pharmaceuticals 13, 34 (2020)
PMID: 32106507 - Shibata N, Ohoka N, Tsuji G, Demizu Y, Akiyama T, *Naito M.
Deubiquitylase USP25 prevents degradation of BCR-ABL protein and ensures proliferation of Ph-positive leukemia cells.
Oncogene 39, 3867-3878 (2020)
PMID: 32203161 - Yokoo H, Ohoka N, Naito M, *Demizu Y.
Design and synthesis of peptide-based chimeric molecules to induce degradation of the estrogen and androgen receptors.
Bioorg. Med. Chem. 28, 115595 (2020)
PMID: 32631565 - Yamamoto J, Suwa T, Murase Y, Tateno S, Mizutome H, Asatsuma-Okumura T, Shimizu N, Kishi T, Momose S, Kizaki M, Ito T, *Yamaguchi Y, *Handa H.
ARID2 is a pomalidomide-dependent CRL4CRBN substrate in multiple myeloma cells.
Nat. Chem. Biol. 16, 1208-1217 (2020)
PMID: 32958952 - *Yamano K, Kikuchi R, Kojima W, Hayashida R, Koyano F, Kawakami J, Shoda T, Demizu Y, Naito M, Tanaka K, Matsuda N.
Critical role of mitochondrial ubiquitination and the OPTN-ATG9A axis in mitophagy.
J. Cell Biol. 219, e201912144 (2020)
PMID: 32556086 - Hirano M, Saito C, Goto C, Yokoo H, Kawano R, *Misawa T, *Demizu Y.
Rational design of helix-stabilized antimicrobial peptide foldamers containing a,a-disubstituted amino acids or side-chain stapling.
ChemPlusChem 85,2731-2736 (2020)
PMID: 33369262 - Yokoo H,# Shibata N,# Naganuma M, Murakami Y, Ito T, *Aritake K, *Naito M, *Demizu Y.
Development of hematopoietic prostaglandin D synthase-degradation inducer.
ACS Med. Chem. Lett. 12,236-241 (2021)
PMID: 33603969
# These authors equally contributed to this work.
<Selected as Supplementary Cover> - Hirano M, Saito C, Yokoo H, Goto C, Kawano R, *Misawa T, *Demizu Y.
Structure-activity relationship analysis of Mag2-based peptide foldamers.
Molecules 26,444 (2021)
PMID: 33466998 - Soma-Kaiho A, Akizuki Y, Igarashi K, Endo A, Kawase Y, Shoda T, Demizu Y, Naito M, Saeki Y, Tanaka K, *Ohtake F.
TRIP12 enhances small molecule-induced degradation of BRD4 through K29/K48 branched ubiquitin chains.
Mol. Cell, 81, 1411-1424.e7 (2021)
PMID: 33567268 - Ichikawa Y, Hiramatsu M, Mita Y, Makishima M, Matsumoto Y, Masumoto Y, Muranaka A, Uchiyama M, Hashimoto Y, *Ishikawa M.
meta-Non-flat substituents: a novel molecular design to improve aqueous solubility in small molecule drug discovery.
Org. Biomol. Chem. 19, 446-456 (2021)
PMID: 33331380 - Tsukumo Y,#Tsuji G,#Yokoo H, Shibata N, Ohoka N, Demizu Y, *Naito M.
Protocols for synthesis of SNIPERs and the methods to evaluate the anticancer effects.
Methods Mol. Biol. 2365, 331-347 (2021)
PMID: 34432253
#These authors equally contributed to this work. - Xu H,#Ohoka N,#Yokoo H, Nemoto K, Ohtsuki T, Matsufuji H, Naito M, Inoue T, *Tsuji G, *Demizu Y.
Development of agonist-based PROTACs targeting liver X receptor.
Front. Chem. 9, 674967 (2021)
PMID: 34124002
#These authors equally contributed to this work. - Nakane K, *Sato S, Niwa T, Tsushima M, Tomoshige S, Taguchi H, Ishikawa M, Nakamura H.
Proximity Histidine Labeling by Umpolung Strategy Using Singlet Oxygen.
J. Am. Chem. Soc. 143, 7726-7731 (2021)
PMID: 33904715 - Morimoto J, Miyamoto K, Ichikawa Y, Uchiyama M, Makishima M, Hashimoto Y, *Ishikawa M.
Improvement in aqueous solubility of achiral symmetric cyclofenil by modification to a chiral asymmetric analog.
Sci. Rep. 11, 12697 (2021)
PMID: 34135380 - *Yokoo H, Hirano M, Ohoka N, Misawa T, *Demizu Y.
Structure-activity relationship study of amphipathic antimicrobial peptides using helix-destabilizing sarcosine.
J. Pept. Sci. e3360, 1-6 (2021)
PMID: 34164880 - Obara S, Nakane K, Fujimura C, Tomoshige S, Ishikawa M, *Sato S.
Functionalization of Human Serum Albumin by Tyrosine Click.
Int. J. Mol. Sci. 22, 8676-8676 (2021)
PMID: 34445381 - Yokoo H,#Ohoka N,#Takyo M, Ito T, Tsuchiya K, Kurohara T, Fukuhara K, Inoue T, Naito M, *Demizu Y.
Peptide stapling improves the sustainability of a peptide-based chimeric molecule that induces targeted protein degradation.
Int. J. Mol. Sci. 22, 8772 (2021)
PMID: 34445478
#These authors equally contributed to this work. - Yokoo H, *Shibata N, Endo A, Ito T, Yanase Y, Murakami Y, Fujii K, Hamamura K, Saeki Y, *Naito M, *Aritake K, *Demizu Y.
Discovery of a highly potent and selective PROTAC targeting hematopoietic prostaglandin D synthase via in silico design.
J. Med. Chem. 64, 15868-15882 (2021)
<Selected as Supplementary Cover> - Shimizu N, Asatuma-Okumura T, Yamamoto J, Yamaguchi Y, *Handa H,*Ito T.
PLZF and its fusion proteins are pomalidomide-dependent CRBN neosubstrates.
Commun Biol. 4, 1277 (2021)
PMID: 34764413 - Naganuma M, *Ohoka N, Tsuji G, Tsujimura H, Matsuno K, Inoue T, Naito M, *Demizu Y.
Development of chimeric molecules that degrade the estrogen receptor using decoy oligonucleotide ligands.
ACS Med. Chem. Lett. 13, 134-139 (2022)
PMID: 35059133
<Selected as Supplementary Cover> - #Ohoka N, #Yokoo H, Okuhira K, Demizu Y, *Naito M.
Molecular design, synthesis and evaluation of SNIPER(ER) that induces targeted protein degradation of ER α.
Methods Mol. Biol. 2418, 363-382 (2022)
PMID: 35119675
#These authors equally contributed to this work. - Hirai K, Yamashita H, *Tomoshige S, Mishima Y, Niwa T, Ohgane K, Ishii M, Kanamitsu K, Ikemi Y, Nakagawa S, Taguchi H, Sato S, Hashimoto Y, *Ishikawa M.
Conversion of a PROTAC mutant huntingtin degrader into small-molecule hydrophobic tags focusing on drug-like properties.
ACS Med. Chem. Lett. 13, 396-402 (2022)
PMID: 35300080 - Shibata N, Cho N, Koyama H, Naito M.
Development of a degrader against oncogenic fusion protein FGFR3-TACC3.
Bioorg Med Chem Lett 60, 128584 (2022)
PMID: 35085722 - Yu S, Wang L, Che D, Zhang M, Li M, Naito M, Xin W, Zhou L.
Targeting CRABP-II overcomes pancreatic cancer drug resistance by reversing lipid raft cholesterol accumulation and AKT survival signaling.
J Exp Clin Cancer Res 41, 88 (2022)
PMID: 35260193 - Nakane K, Niwa T, Tsushima M, Tomoshige S, Taguchi H, Nakamura H, Ishikawa M, Sato S.
BODIPY Catalyzes Proximity‐Dependent Histidine Labelling.
ChemCatChem 14, e202200077 (2022) - Nakano N, Fukuda K, Tashiro E, Ishikawa H, Nagano W, Kawamoto R, Mori A, Watanabe M, Yamazaki R, Nakane T, Naito M, Okamoto I, *Itoh, S.
Hybrid molecule between platanic acid and LCL-161 as a yes-associated protein degrader.
J Biochem. 171, 631-640 (2022)
PMID: 35211741 - #Xu H, #Kurohara T, Takano R, Yokoo H, Shibata N, Ohoka N, Inoue T, Naito M, *Demizu Y.
Development of rapid and facile solid-phase synthesis of PROTACs via a variety of binding styles.
ChemistryOpen. 11, e202200131 (2022) #These authors equally contributed to this work.
PMID: 35822913 - Takada H, Tsuchiya K, *Demizu Y.
Helix-stabilized cell-penetrating peptides for delivery of antisense morpholino oligomers: Relationships among helicity, cellular uptake, and antisense activity.
Bioconjug. Chem. 33, 1311-1318 (2022)
PMID: 35737901
<Selected as Front Cover> - Ohoka N, Suzuki M, Uchida T, Tsukumo Y, Yoshida M, Inoue T, Ohki H, *Naito M.
Development of a potent small-molecule degrader against oncogenic BRAF(V600E) protein that evades paradoxical MAPK activation.
Cancer Sci. 113, 2828-2838 (2022)
PMID: 35579105 - #Murakami Y, #Osawa H, #Kurohara T, Yanase Y, Ito T, Yokoo H, Shibata N, Naito M, Aritake K, *Demizu Y.
Structure-activity relationship study of PROTACs against hematopoietic prostaglandin D2 synthase.
RSC Med. Chem. 13, 1495-1503 (2022)
PMID: 36561070
#These authors equally contributed to this work. <Selected as Front Cover> - Nakane K, Nagasawa H, Fujimura C, Koyanagi E, Tomoshige S, Ishikawa M, Sato S.
Switching of Photocatalytic Tyrosine/Histidine Labeling and Application to Photocatalytic Proximity Labeling.
Int. J. Mol. Sci. 23, 11622 (2022)
PMID: 36232972 - #Yokoo H, #Misawa T, Kato T, Tanaka M, *Demizu Y, *Oba M.
Development of delivery carriers for plasmid DNA by conjugation of a helical template to oligoarginine.
Bioorg. Med. Chem. 72, 116997 (2022)
PMID: 36088811
#These authors equally contributed to this work. - Akizuki Y, Morita M, Mori Y, Kaiho-Soma A, Dixit S, Endo A, Shimogawa M, Hayashi G, Naito M, Okamoto A, Tanaka K, Saeki Y, *Ohtake F.
cIAP1-based degraders induce degradation via branched ubiquitin architectures.
Nat Chem Biol. 19, 311-322 (2022)
PMID: 36316570 - Tsuchiya K, Kiyoshi M, Hashii N, Fujita M, Kurohara T, Ishii-Watabe A, Fukuhara K, *Misawa T, *Demizu Y.
Development of a penetratin-conjugated stapled peptide that inhibits Wnt/β-catenin signaling.
Bioorg. Med. Chem. 73, 117021 (2022)
PMID: 36198218 - Ohoka N, Suzuki M, Uchida T, Tsuji G, Tsukumo Y, Yoshida M, Inoue T, Demizu Y, Ohki H, *Naito M.
Development of Gilteritinib-Based Chimeric Small Molecules that Potently Induce Degradation of FLT3-ITD Protein.
ACS Med. Chem. Lett. 13, 1885-1891 (2022)
PMID: 36518702 - Takyo M, Sato Y, Hirata N, Tsuchiya K, Ishida H, Kurohara T, Yanase Y, Ito T, Ito N, Kanda Y, Yamamoto K, *Misawa T, *Demizu Y.
Oligoarginine-conjugated peptide foldamers inhibiting vitamin D receptor-mediated transcription.
ACS Omega. 7, 46573-46582 (2022)
PMID: 36570290
Former Publications
- Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, *Handa H.
Identification of a primary target of thalidomide teratogenicity.
Science 327, 1345-1350 (2010)
PMID: 20223979 - Itoh Y, Ishikawa M, Naito M, *Hashimoto Y.
Protein knockdown using methyl bestatin-ligand hybrid molecules: design and synthesis of inducers of ubiquitination-mediated degradation of cellular retinoic acid-binding proteins.
J. Am. Chem. Soc. 132, 5820-5826 (2010)
PMID: 20369832 - Matyskiela ME, *Lu G, Ito T (co-1st author), Pagarigan B, Lu C-C, Miller K, Fang W, Wang NY, Nguyen D, Houston J, Carmel G, Tran T, Riley M, Nosaka L, Lander GC, Gaidarova S, Xu S, Ruchelman AL, Handa H, Carmichael J, Daniel TO, Cathers BE, Lopez-Girona A, *Chamberlain PP.
A novel cereblon modulator recruits GSPT1 to the CRL4CRBN ubiquitin ligase.
Nature 535, 252-257 (2016)
PMID: 27338790 - Yamashita H, Kato T, Oba M, Misawa T, Hattori T, Ohoka N, Tanaka M, Naito M, *Kurihara M, *Demizu Y.
Development of a cell-penetrating peptide that exhibits responsive changes in its secondary structure in the cellular environment.
Sci. Rep. 6, 33003 (2016)
PMID: 27609319 - Tomoshige S, Nomura S, Ohgane K, Hashimoto Y, *Ishikawa M.
Discovery of small molecules that induce the degradation of huntingtin.
Angew. Chem. Int. Ed. 56, 11530-11533 (2017)
PMID: 28703441 - Okuhira K, Shoda T, Omura R, Ohoka N, Hattori T, Shibata N, Demizu Y, Sugihara R, Ichino A, Kawahara H, Itoh Y, Ishikawa M, Hashimoto Y, Kurihara M, Itoh S, Saito H, *Naito M.
Targeted degradation of proteins localized in subcellular compartments by hybrid small molecules.
Mol. Pharmacol. 91, 159-166 (2017)
PMID: 27965304 - Ohoka N, Okuhira K, Ito M, Nagai K, Shibata N, Hattori T, Ujikawa O, Shimokawa K, Sano O, Koyama R, Fujita H, Teratani M, Matsumoto H, Imaeda Y, Nara H, Cho N, *Naito M.
In vivo knockdown of pathogenic proteins via specific and nongenetic inhibitor of apoptosis protein (IAP)-dependent protein erasers (SNIPERs).
J. Biol. Chem. 292, 4556-4570 (2017)
PMID: 28154167 - Mori T, Ito T (co-1st author), Liu S, Ando H, Sakamoto S, Yamaguchi Y, Tokunaga E, Shibata N, *Handa H, *Hakoshima T.
Structural basis of thalidomide enantiomer binding to cereblon.
Sci. Rep. 8, 1294 (2018)
PMID: 29358579 - Okitsu K, Hattori T, Misawa T, Shoda T, Kurihara M, *Naito M, *Demizu Y.
Development of a small hybrid molecule that mediates degradation of His-tag fused proteins.
J. Med. Chem. 61, 576-582 (2018)
PMID: 28460164 - Shibata N, Nagai K, Morita Y, Ujikawa O, Ohoka N, Hattori T, Koyama R, Sano O, Imaeda Y, Nara H, Cho N, *Naito M.
Development of protein degradation inducers of androgen receptor by conjugation of androgen receptor ligands and inhibitor of apoptosis protein ligands.
J. Med. Chem. 61, 543-575 (2018)
PMID: 28594553 - Nishiyama Y, Mori S, Makishima M, Fujii S, Kagechika H, Hashimoto Y, *Ishikawa M.
Novel nonsteroidal progesterone receptor (PR) antagonists with a phenanthridinone skeleton.
ACS Med. Chem. Lett. 9, 641-645 (2018)
PMID: 30034593





